Translate this page into:
Incidence and predictors of death among adult patients treated for tuberculosis in two regions of Cameroon: 2010 to 2015
*Corresponding author: Adamou Dodo Balkissou, Department of Medicine, Faculty of Medicine and Biomedical Sciences of Garoua, University of Ngaoundere, Garoua, Cameroon. dodobalkissou@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Balkissou AD, Pefura-Yone EW, Poka V, Kuaban A, Mubarak DM, Kora AD, et al. Incidence and predictors of death among adult patients treated for tuberculosis in two regions of Cameroon: 2010 to 2015. J Pan Afr Thorac Soc 2022;3:34-41.
Abstract
Objectives:
Mortality during tuberculosis (TB) remains high in Africa. The purpose of our study was to determine the incidence and predictors of death during TB treatment in Cameroon.
Materials and Methods:
Data of subjects aged ≥15 years were retrospectively extracted from registers in all TB diagnostic and treatment centers in the Western and Northern regions of Cameroon from 2010 to 2015. Cox regression models were used to determine predictors of death during TB treatment.
Results:
Of the 19,681 patients included, 12,541 (63.7%) were male and their median age (25th-75th percentile) was 34 (26–45) years. The cumulative incidence (95% confidence interval [CI]) of death during TB treatment was 8.0% (7.5–8.5%). The predictors (hazard ratios [95% CI]) of death were: Age >34 years (1.479 [1.295–1.688], P < 0.001), female sex (1.471 [1.286–1.683], P < 0.001), extra-pulmonary TB (1.723 [1.466–2.026], P < 0.001), human immunodeficiency virus infection (3.739 [3.269–4.276], P < 001]), TB treatment in the Western region (2.241 [1.815–2.768], P < 0.001), treatment before 2012 (1.215 [1.073–1.376], P = 0.002)and low body weight (1st quartile and 2nd quartile) (2.568 [2.133–3.092], [P < 0.001]) and (1.569 [1.298–1.896], P < 0.001) respectively.
Conclusion:
The incidence of death during TB was relatively high in the Western and Northern regions of Cameroon. Recognition of these persons at greatest risk may improve care and reduce death durinng TB treatment.
Keywords
Death
Tuberculosis
Incidence
Predictors
Cameroon
INTRODUCTION
Tuberculosis (TB) is one of the top ten causes of mortality in the world according to the World Health Organization (WHO).[1] In 2019, an estimated 10.0 million (range, 8.9 – 11.0 million) people contracted TB and 1.2 million (range, 1.1 – 1.3 million) also died from it, and an additional 208,000 (range, 177,000–242,000) with the human immunodeficiency virus (HIV).[2] The actual WHO strategic plan for TB is to reduce the number of deaths by 95% and the incidence of TB by 90% from 2015 to 2035.[3] In Cameroon, 24,740 cases of TB were reported in 2019, 27% of whom were people living with HIV, with 95% of them on antiretroviral therapy and the incidence rate of HIV/TB coinfection was estimated at 48 (31–69) per 100,000 people in 2019.[4] In the same year, the number of deaths due to TB was estimated at 7400 (4400–11,000) in HIV-negative subjects and 5000 (3200–7,200) in people living with HIV. An Ethiopian meta-analysis of 34 studies conducted from 2003 to 2016 found 8% of deaths during TB treatment.[5] In some African countries, the mortality rate during TB was high, reaching 10% in Ghana, 16% in Nigeria, and 20% in Southwest Ethiopia.[6-8] Predictors of death were TB/HIV coinfection, severe immunodeficiency, smear-negative pulmonary TB, low weight, and malnutrition.[9-11] Since 2008, many actions have been taken by the national TB program and stakeholders in many African countries, to improve decentralization of TB treatment and community TB treatment care, to reduce lost to follow-up and death due to TB.[12] Previous studies on TB mortality in Cameroon found HIV as the main risk factor for death during TB.[10,13,14] From 1996 to 2011 in the West Region of Cameroon, 8.6% of patients died during TB treatment and 36.5% of deaths occurred during the first 2 months of TB treatment. HIV infection, extra-pulmonary TB, sputum smear-negative pulmonary TB, and male sex were significant independent risk factors of death.[10] The prevalence of HIV infection was 35% on 1419 adult patients treated for TB in 2009 and the mortality rate was 10.5% in HIV positive patients. In the same year, death rate was 3.7% among those with CD4 above 200/mm(3) and 13% among those with CD4 below 200/ mm(3).[13] The prevalence of HIV in Cameroon declined from 5.1% in 2010 to 4.7% in 2015.[15] Similarly, HIV testing among patients with TB increased from 76% in 2010 to 93% in 2015,[15] and approximately 90% of HIV positive patients were on antiretroviral therapy. Knowing however that the incidence of TB/HIV coinfection had reduced in the last decades both worldwide and in Cameroon,[4] and the number of HIV patients on antiretroviral therapy had increased, we wanted to provide an update on the risk factors of death during TB. We therefore carried out this study with the aim to determine the incidence and predictors of death during TB treatment in two regions of Cameroon.
MATERIALS AND METHODS
Design and study population
The study population consisted of a retrospective cohort of all consecutive TB patients aged ≥ 15 years who were put on anti-TB treatment between January 2010 and December 2015 in all the TB diagnostic and treatment centers (DTC) of the West and North Regions of Cameroon. Each subject was retrospectively followed over an 8-month period for new cases and 10 months for retreatment cases. We excluded patients with missing data on age, sex, and outcome.
Study setting
Cameroon is a Central African country with an estimated population of 24,910,305 inhabitants in 2020.[16] During the study period, there were 20 operational DTC in the Western region and 17 operational DTC in the Northern Region. The study was conducted in all the TB DTC of the two regions. According to the health pyramid, each region had only one DTC at the regional hospital (third category) and other DTCs were located in the district hospital (fourth category). In these DTC and in accordance with international guidelines and National Tuberculosis Control Program guidelines of Cameroon,[17] pulmonary TB and extra-pulmonary TB was diagnosed after clinical examination including anthropometric measurements (weight and height), microscopy (sputum smear), and other paraclinical exams. TB management was the same in all DTCs in Cameroon during the study period.[17] The drugs were available and dispensed free of charge in DTC. Antiretroviral drugs recommended for all patients with HIV were available and given free of charge in all DTC of Cameroon.
Each patient with TB was classified and treated according to bacteriological status (smear positive or negative pulmonary TB), disease location (pulmonary or extrapulmonary TB), and therapeutic history (new case or retreatment [relapses, failures, and lost to follow-up]). The new cases were defined as patients who had never been previously treated with anti-TB drugs (or treated for <1 month).[17] Relapses were patients who currently had smear positive pulmonary TB, but who had previously been treated for active TB (bacteriologically confirmed or not) and who had been declared “cured” or “treatment completed” after complete TB chemotherapy. Failures were represented by patients undergoing treatment who had a positive microscopy during sputum smear control of the 5th month or later during treatment. The defaulters were defined as those who took TB treatment for a month or more and who, having discontinued this treatment for at least 2 months, present with symptoms of pulmonary TB and positive sputum tests. Those who have a negative sputum test when they return must complete the duration of the treatment initially prescribed to them.
Therapeutic regimens always consisted of two phases: An intensive initial phase and a continuation phase. During the intensive phase that lasted 2 months for new cases and 3 months for retreatment cases, the drugs used were: Rifampicin, Isoniazid, Ethambutol, Pyrazinamide, and Streptomycin which was associated for retreatment cases. During the continuation phase that lasted 4 months for new cases, 5 months for retreatment cases, and 10 months for certain particular forms (meningitis, Pott’s disease); the drugs used were: Rifampicin, Isoniazid, to which Ethambutol was added in case of retreatment. Treatment could be taken entirely as an outpatient or hospitalized during the intensive phase for patients with altered general states. Treatment should be taken on a daily and regular basis until its completion.[17] Antiretroviral therapy was recommended for any HIV-positive patient with active TB regardless of the level of lymphocytes cluster differentiation (CD) type 4. Bacteriological controls were carried out periodically (end of 2nd month, 5th month, 6th month, or 8th month) to monitor the patient’s progress during smear positive pulmonary TB treatment. The treatment outcomes of patients were recorded as: Cured, completed treatment, failure, death, lost to follow-up, and transfer. Those patients, whose sputum smear was negative during the last month of treatment and on at least one previous occasion, were defined as “cured.” Treatment completed was defined for patients who finished treatment but did not meet the criteria for being classified as “cured” or “failed.” “Failures” were patients whose smears of sputum were positive at the 5th month or later during well-conducted treatment. “Death” was defined as any patient who died for any reason during the course of the treatment for TB. Patients “lost to follow-up” were those whose treatment was interrupted for 2 consecutive months or more during the treatment period.
Data collection
Data were extracted from TB treatment registers and treatment forms and registered in an electronic questionnaire previously developed in Epidata version 3.1 (Lauritzen, Denmark). At baseline, data were collected on the different variables that are useful for the study and then the outcomes. The patient’s socio-demographic data were: Age, gender, place of residence, and place of treatment. The clinical baseline data were: Weight in Kilograms (Kg), type of TB (new cases or cases of relapse, failure, and lost to follow-up), form of TB (smear positive or negative pulmonary TB and extra-pulmonary TB), and the specific location of TB for extra-pulmonary TB. The biological data were sputum test results at the beginning of treatment, HIV status, and CD4 lymphocytes count. The treatment outcomes of patients were recorded as: Cured, completed treatment, failure, death, lost to follow-up, and transfer.
Patient and public involvement statement
This retrospective cohort did not involve patients in the development of the research question, in the design, in the recruitment, and conduct of the study. Thus, the results could not be disseminated to the study participants.
Ethical issues
Ethical clearance for the study was obtained from the Institutional Review Board of the Faculty of Medicine and Biomedical Sciences of the University of Yaounde I, ID approval N° 0255/UY1/FMSB/VDRC/CSD and from the Faculty of Medicine and Pharmaceutical Sciences of the University of Douala, ID approval N°CEIUD/192/02/2015/T, N°CEI-Udo/1075/07/2017/T and N°1473/CEI-UDo/04/2018/T.
Administrative authorization to carry out the study at the TB DTC was granted by the authorities of the regional delegation of public health with the following ID approval number N°89/L/MINSANTE/ST/PNLT/GTC/DRSP/URTL_ CE, N°0386/NS/D/DRSPN/GRA, N°125/L/MINSANTE/SG/ DRSPO/CBF and N°126/L/MINSANTE/SG/DRSPO/CBF.
Data analysis
Data analysis was done using the Statistical Package for the Social Science (SPSS) version 23 software for Windows (IBMSPSS Inc., Chicago, US). The variables were summarized using frequencies. The Chi-squared test or where appropriate the exact Fisher test was used to compare proportions while the Student t-test or its non-parametric equivalent test was used for comparison of quantitative variables. For the analyses, follow-up time was time from treatment start to date of outcome which was presented into person-months (pm) at risk. The incidence density was calculated as follows.
Kaplan–Meier analysis and log rank test (Mantel-Cox) were used to compare survival curves stratified by gender, previous TB treatment, type of TB, and HIV status. Deaths were compared to the rest of the study population (survivors = cured + completed + failed + defaulters + transferred). To estimate hazard ratios (HR) with corresponding 95% confidence intervals (CI), Cox proportional hazards modeling was done and used to determine risk factors for death. Covariates that were associated with each outcome measure with P < 0.10 were included in the multivariable analysis. Difference was considered to be significant if P < 0.05.
RESULTS
General characteristics of the study population
A total of 19,958 patient’s records were reviewed, and 277 (1.4%) incomplete records were excluded. Of the 19,681 patient’s records included in the study, 12,541 (63.7%) were male and 7140 (36.3%) were female subjects. The median age (25th–50th percentile) was 34 (26–45) years ranging from 15 to 120 years [Table 1]. Retreatment was observed in only 4.6% cases. Pulmonary TB was noted in 88.5% cases with smear negative pulmonary TB found in 10.6% of our study population. Pleural TB was the most frequent extra pulmonary localization as seen in 28.1% of cases, followed by lymph node TB and Pott’s disease in 20.3% and 11.1% of cases, respectively. HIV serology was positive in 5,167/17,691 (29.2%) patients who did the test.
| Characteristics | n=19,681 (%) |
|---|---|
| Age, years | |
| ≤34 | 9882 (50.2) |
| >34 | 9799 (49.8) |
| Minimum-Maximum | 15–120 |
| Gender | |
| Female | 7140 (36.3) |
| Male | 12,541 (63.7) |
| Region of registration | |
| West | 7,928 (40.3) |
| North | 11,753 (59.7) |
| Year of registration | |
| ≤2012 | 9,900 (50.3) |
| >2012 | 9,781 (49.7) |
| Type of patients | |
| Retreatment | 895/19,499 (4.6) |
| New cases | 18,604/19,499 (95.4) |
| Referred patients | |
| Yes | 429/19,671 (2.2) |
| No | 19,242/19,671 (97.8) |
| Weight (Kg) | |
| Median (IQR) | 56 (14) |
| <25th percentile | 2067/8227 (25.1) |
| 25th –50th percentile | 1957/8227 (23.8) |
| 50th –75th percentile | 2170/8227 (26.4) |
| >75th percentile | 2033/8227 (24.7) |
| Form of tuberculosis | |
| EPTB | 2261 (11.5) |
| PTB | 17,420 (88.5) |
| Pulmonary tuberculosis | |
| SNPTB | 1845/17,416 (10.6) |
| SPPTB | 15,571/17,416 (89.4) |
| Initial microscopy | |
| ≥2 + | 8666/15,528 (55.8) |
| <2 + | 6862/15,528 (44.2) |
| HIV status | |
| Positive | 5167/17,691 (29.2) |
| Negative | 12,524/17,691 (70.8) |
| CD4/mm3, median (IQR) | 150 (222) |
| CD4<150/mm3 | 217/435 (49.9) |
| CD4≥150/mm3 | 218/435 (50.1) |
| Outcomes | |
| Cured | 11,505 (58.5) |
| Completed treatment | 5,425 (27.6) |
| Death | 1,565 (8.0) |
| Failure | 222 (1.1) |
| Lost to follow-up | 560 (2.8) |
| Transfer | 404 (2.1) |
CI: Confidence interval, IQR: Interquartile range, PTB: Pulmonary tuberculosis, EPTB: Extra-pulmonary tuberculosis, SNPTB: Smear negative pulmonary tuberculosis, SPPTB: Smear positive pulmonary tuberculosis, HIV: Human immunodeficiency virus, CD4: Cluster differentiation 4, Kg: Kilogram
Outcomes of TB
The incidence (95% CI) of poor outcomes was represented, respectively, by 8.0% (7.5–8.5%) deaths, 2.8% (2.6–3.1%) lost to follow-up, and 1.1% (1.0–1.3%) failure to treatment. Four hundred and four [2.1% (1.9–2.3%)], patients were transferred out. Treatment success was observed in 86.0% of our study population, with 58.5% (57.7–59.1%) cured patients and 27.6% (26.9–29.2%) patients who completed their treatment [Table 1].
Incidence of death among patients treated for TB
During a total follow-up time of 109,328 person-months in 19,681 patients, 1565 persons died, giving a mortality incidence density of 1.43 (95% CI: 1.36–1.50) per 100 person-months. There was a reduction of the cumulative death incidence from 9.9% in 2010 to 6.8% in 2015, P = 0.003. As presented in [Figure 1], the incidence of death was consistently higher in West region compared to North region between 2010 and 2015. Incidence of death was higher in patients recruited in the West region compared to those recruited in the North region (15.7% vs. 3.1%, P < 0.001). Analysis of mortality rate showed that most deaths occurred within the 1st month of treatment, with a crude mortality rate of 50.5% (791/1565 patients) as compared to the 226 (14.4%) patients in the 2nd month, 138 (8.8%) patients in the 3rd month, and 410 (26.2%) patients in the 4th month and thereafter. The median (25th-75th percentile) time of death was 30 (8–98) days.
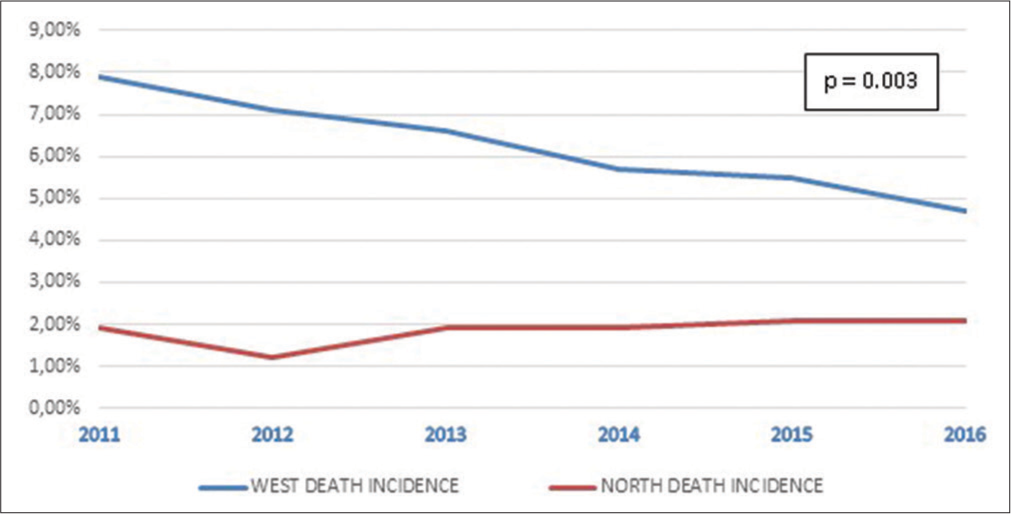
- Evolution of tuberculosis mortality rate from 2010 to 2015 in West and North Region of Cameroon.
Predictors of death among patients treated for TB
Kaplan-Maier analysis and Log Rank (Mantel-Cox) test show differences in mortality between sub-groups [Figure 2] and [Figure 3]. When stratified by type of TB, mortality was higher for patients with EPTB [Figure 2] (P < 0.001). When stratified by HIV status, mortality was lowest among HIV negative patients [Figure 3] (P = 0.001). When stratified by age, mortality was higher among patients aged >34 years (P = 0.001). There were no statistical differences after stratification by gender (P = 0.053) and previous TB treatment (P = 0.119).

- Death probability during tuberculosis (TB) treatment according to type of TB.

- Death probability during tuberculosis treatment according to the HIV status.
[Table 2] shows factors associated with death during TB treatment after Cox regression. In univariate analysis, the factors (Hazards ratio [HR] [95% CI]) associated with death during TB treatment were patients recruited before 2012 compared to those recruited after 2012 (P < 0.001). Patients who died during TB treatment were older with a median age (IQR) of 39 (11) years (HR [95% CI], 1.888 [1.702–2.095], P < 0.001]). Patients with the lowest weight included in the 1st quartile died the most during TB treatment (1.779 [1.514– 2.137], P < 0.001). Extra-pulmonary TB (1.713 [1.506–1.949], P < 0.001s), low sputum smear (bacilli load ≥2+: 0.632 [0.565–0.707], P < 0.001), and referred patients (2.050 [1.271–3.306], P = 0.003) were associated with death. Positive HIV status was associated with death during TB (4.557 [4.090–5.078], P < 0.001). A reduction of the CD4 count was associated with death during TB (0.0310 [0.206–0.466], P < 0.001). Retreatment cases (1.069 [0.848–1.349], P = 0571) were not associated with death in our study.
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR | (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, years, median (years) | |||||
| >34 | 1.888 | (1.702–2.095) | <0.001 | 1.479 (1.295–1.688) | <0.001 |
| ≤34 | 1 | ||||
| Gender | |||||
| Female | 1.101 | (0.994–1.219) | 0.064 | 1.471 (1.286–1.683) | <0.001 |
| Male | 1 | ||||
| Region of registration | |||||
| West | 0.192 | (0.170–0.216) | <0.001 | 2.241 (1.815–2.768) | <0.001 |
| North | 1 | ||||
| Year of registration | |||||
| ≤2012 | 0.822 | (0.744–0.908) | <0.001 | 1.215 (1.073–1.376) | 0.002 |
| >2012 | 1 | ||||
| Form of TB | |||||
| EPTB | 1.713 | (1.506–1.949) | <0.001 | 1.723 (1.466–2.026) | <0.001 |
| PTB | 1 | ||||
| Weight (Kg) | 0.980 | (0.974–0.986) | <0.001 | ||
| <25th percentile | 1.799 | (1.514–2.137) | <0.001 | 2.568 (2.133–3.092) | <0.001 |
| 25th –50th percentile | 1.339 | (1.118–1.604) | 0.002 | 1.569 (1.298–1.896) | <0.001 |
| 50th –75th percentile | 1.104 | (0.919–1.326) | 0.289 | 1.198 (0.991–1.448) | 0.062 |
| >75th percentile | 1 | 1 | |||
| HIV infection | 4.557 | (4.090–5.078) | <0.001 | 3.739 (3.269–4.276) | <0.001 |
| CD4 count/mm3 | 0.310 | (0.206–0.466) | <0.001 | ||
HR: Hazards ratio, CI: Confidence interval, IQR: Interquartile range, TB: Tuberculosis, PTB: pulmonary tuberculosis, EPTB: Extra-pulmonary tuberculosis, HIV: Human immunodeficiency virus, CD4: Cluster differentiation 4, BMI: Body mass index, Kg: kilogram, m2: Meter2
After multivariate analysis [Table 2], the predictors were: Age >34 years (1.479 [1.295–1.688], P < 0.001), female gender (1.471 [1.286–1.683], P < 0.001), extra-pulmonary TB (1.723 [1.466–2.026], P < 0.001), HIV infection (3.739 [3.269–4.276], P < 001), recruitment in the West region (2.241 [1.815–2.768], P < 0.001), treatment before 2012 (1.215 [1.073–1.376]), P = 0.002, and low weight (1st quartile and 2nd quartile) (2.568 [2.133–3.092], [P < 0.001] and 1.569 [1.298–1.896], P < 0.001), respectively.
DISCUSSION
The objective of this study was to determine the incidence and predictors of death during TB in two regions of Cameroon. The cumulative incidence of TB death was 8.0%. The predictors of death during TB were: Age, female sex, patients recruited in West Region before 2012, HIV infection, extra-pulmonary TB, and low body weight.
The incidence of death during TB in our study was in the range of 5.0%–9.5% found in literature.[5,11,18-22] Eshetie et al. in the meta-analysis of 34 Ethiopian studies conducted from 2003 to 2016 reported 8.0%[5] and Ejeta et al. reported a rate of 8.1%,[23] as in our study. The incidence of death reached 11.3% in Ukraine,[24] 13.5% in Ghana,[6] 16.6% in Nigeria,[7] and 20.2% in Southwest Ethiopia,[8] which was higher than the rate found in our cohort. This could be due to the increase in the prevalence of multidrug-resistant TB and HIV infection in recent decades.[5,25] In Nigeria, the proportion of case fatality was well above twice that found in our cohort. This could be explained by the high proportion of TB/HIV coinfection which was 39.8% (with only 6.3% on ART) compared to 29.2% (up to 46.1% of them on ART in 2009[14]) in our cohort and also due to Boko Haram insurgency in Kano state, where that study was done, leading to a large number of displaced and vulnerable populations.[7,26] In Cameroon HIV positive patients, the death rate was 3.7% among those with CD4 above 200/mm3 and 13% among those with CD4 below 200/mm3 (P < 0.02).[13] On the other hand, the incidence of death was low in Turkey (2.4%) compared to our study.[27] This could be explained by the differences in the study population, which was only patients with bacteriologically confirmed pulmonary TB, with 92.6% successful treatment outcome. In that Turkish study, treatment outcome was successful in young and educated pulmonary TB patients who had no drug resistance, no previous treatment history, and no comorbidities.
Predictors of death during TB found in our study were: Age, female sex, recruitment before 2012 in the West region, extra-pulmonary TB, people living with HIV, and underweight. Dangisso et al. revealed that people over the age of 34 were twice as likely to die from TB.[28] These results are similar to those of our study, in which the median age was 34 years. This may be due to the increase of comorbidities with age as it was reported in that Ethiopian study. Studies carried out in South Africa and Southwest Ethiopia found that female sex was associated with death during TB from 2010 to 2012 as in our study.[8,20] Comparable results were described in South Asia and Zambia.[29,30] Low body mass indexes were factors associated with death during TB after multivariate analysis, as found in our study.[19,31,32] This could be due to an advanced disease caused by delay in diagnosis related to poor access and utilization of healthcare, poor socio-economic status and social behaviors of women. Notice that 60% of our study population were registered in the North of Cameroon where climate was worse and malnutrition was highest. Regarding HIV, this result was similar to the findings of many other studies.[5,18,26] Ogyiri et al. in Ghana found that people living with HIV (21.8% vs. 5.5%) were more likely to die during TB.[33] The severity of immunodeficiency among TB-HIV coinfected patients has been described as a predictor of decreased survival and was likely the main determinant of early death in HIV-infected PTB patients despite their use of antiretroviral therapy.[13,34] As previously shown in another Cameroonian study, HIV infection is a determinant of extrapulmonary TB.[35] Kapata et al. also found that determinants of TB/HIV coinfection were female sex and extrapulmonary TB.[29] The diagnosis of EPTB was difficult to confirm in regional facilities where there was lack of medical doctors and specialists in our setting. We could easily have differential diagnosis such as cancer and other opportunistic diseases being treated as TB, which resulted certainly to death without adequate treatment, and then registered as TB death incorrectly.
Being diagnosed with TB before 2012 was a predictor of death, while the case fatality decreased progressively from 2010 to 2015 in our study. We speculated that TB patients diagnosed after 2012 died less than those diagnosed before, certainly because of the decrease of HIV infection since 2000 and the systematic use of antiretroviral therapy for people living with HIV since 2013 in Cameroun.[36] The prevalence of HIV in Cameroon was 5.1% in 2010 and 4.8% in 2016.[15,37] Cameroon notified 89,445 persons on ART in 2010, 122,783 people on ART in 2012 and 168,431 persons on ART in 2015.[15] In other words, the number of people on ART approximately doubled during our study period.
Some limitations of our study include the missing data, which is expected for a study with data collected from registers. Missing data on height did not permit us to assess the nutritional status of the study population. This retrospective study did not allow us to obtain specific socioeconomic, demographic, clinical, radiological, and biological information that could possibly predict mortality during TB treatment. The main strength of our study lies in its large sample size which increased our statistical power to reliably characterize the predictors of mortality.
CONCLUSION
The incidence (95% CI) of death during TB was 8.0% (7.5–8.5%) in two regions of Cameroon. The density incidence of death among patients treated for TB was 1.43 (95% CI: 1.36–1.50) per 100 person-months. Predictors of death among patients treated for TB were: Age >34 years, female gender, extra-pulmonary TB, HIV infection, recruitment in the West region, treatment before 2012, and low weight (1st quartile and 2nd quartile). Early detection and adequate management of people living with HIV would reduce mortality during TB.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Tuberculose. 2020. Geneva: World Health Organization; Avaiable from: https://www.who.int/fr/news-room/fact-sheets/detail/tuberculosis [Last accessed on 2020 May 09]
- [Google Scholar]
- OMS Stratégie de l'OMS Pour Mettre Fin à la Tuberculose D'ici 2035. 2016. Geneva: World Health Organization; Avaiable from: https://www.who.int/tb/strategy/fr [Last accessed on 2019 Feb 25]
- [Google Scholar]
- TB Profile. 2021. Geneva: World Health Organization; Avaiable from: https://www.worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22en%22&iso2=%22cm%22 [Last accessed on 2021 May 20]
- [Google Scholar]
- Tuberculosis treatment outcomes in Ethiopia from 2003 to 2016, and impact of HIV co-infection and prior drug exposure: A systematic review and meta-analysis. PLoS One. 2018;13:e0194675.
- [CrossRef] [PubMed] [Google Scholar]
- Reflecting on tuberculosis case notification and treatment outcomes in the Volta region of Ghana: A retrospective pool analysis of a multicentre cohort from 2013 to 2017. Glob Health Res Policy. 2019;4:37.
- [CrossRef] [PubMed] [Google Scholar]
- High mortality among tuberculosis patients on treatment in Nigeria: A retrospective cohort study. BMC Infect Dis. 2017;17:170.
- [CrossRef] [PubMed] [Google Scholar]
- The role of social determinants on tuberculosis/HIV co-infection mortality in southwest Ethiopia: A retrospective cohort study. BMC Res Notes. 2016;9:89.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15:871-85.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of death among tuberculosis patients in a semi urban diagnostic and treatment centre of Bafoussam, West Cameroon: A retrospective case-control study. Pan Afr Med J. 2015;22:253.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a prognostic score during tuberculosis treatment. BMC Infect Dis. 2017;17:251.
- [CrossRef] [PubMed] [Google Scholar]
- Reflections on tuberculosis diagnosis and treatment outcomes in Ghana. Arch Public Health. 2013;71:22.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of the HIV infection on the evolution of tuberculosis among adult patient in Yaounde, Cameroon. Rev Pneumol Clin. 2012;68:338-44.
- [CrossRef] [PubMed] [Google Scholar]
- HIV testing, HIV status and outcomes of treatment for tuberculosis in a major diagnosis and treatment centre in Yaounde, Cameroon: A retrospective cohort study. BMC Infect Dis. 2012;12:190.
- [CrossRef] [PubMed] [Google Scholar]
- Rapport Annuel 2016 des Activités de Lutte Contre le VIH. le SIDA et Les IST au Cameroun: 2016.
- [Google Scholar]
- BUCREP-Accueil. 2021. Avaiable from: http://www.bucrep.cm/index.php/fr [Last accessed on 2021 May 20]
- [Google Scholar]
- Determinants of tuberculosis treatment outcome under directly observed treatment short courses in Adama city, Ethiopia. PLoS One. 2020;15:e0232468.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur District, South India, 2000. Int J Tuberc Lung Dis. 2002;6:780-8.
- [Google Scholar]
- The complex relationship between human immunodeficiency virus infection and death in adults being treated for tuberculosis in Cape Town, South Africa infectious disease epidemiology. BMC Public Health. 2015;15:556.
- [CrossRef] [PubMed] [Google Scholar]
- HIV testing, HIV status and outcomes of treatment for tuberculosis in a major diagnosis and treatment centre in Yaounde, Cameroon: A retrospective cohort study. BMC Infect Dis. 2012;12:190.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment outcomes of tuberculosis patients under directly observed treatment short-course at Debre Tabor General Hospital, Northwest Ethiopia: Nine-years retrospective study. Infect Dis Poverty. 2018;7:16.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of tuberculosis patients under directly observed short course treatment in Western Ethiopia. J Infect Dev Ctries. 2015;9:752-9.
- [CrossRef] [PubMed] [Google Scholar]
- Patient predictors of poor drug sensitive tuberculosis treatment outcomes in Kyiv Oblast, Ukraine. F1000Res. 2017;6:1873.
- [CrossRef] [PubMed] [Google Scholar]
- Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575-80.
- [CrossRef] [Google Scholar]
- The impact of rural residence and HIV infection on poor tuberculosis treatment outcomes in a large urban hospital: A retrospective cohort analysis. Int J Equity Health. 2018;17:4.
- [CrossRef] [PubMed] [Google Scholar]
- Factors affecting successful treatment outcomes in pulmonary tuberculosis: A single-center experience in Turkey, 2005-2011. J Infect Dev Ctries. 2015;9:821.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome of smear-positive tuberculosis patients after initiation and completion of treatment: A ten-year retrospective cohort study. PLoS One. 2018;13:e0193396.
- [CrossRef] [PubMed] [Google Scholar]
- The social determinants of tuberculosis and their association with TB/HIV co-infection in Lusaka, Zambia. 2013. Med J Zambia. 40:49-54. Avaiable from: https://www.ajol.info/index.php/mjz/article/view/110518 [Last accessed on 2020 Oct 03]
- [Google Scholar]
- Understanding the social determinants of TB and HIV in South Asia. PeerJ Prepr. 2014;2:e579.v1.
- [CrossRef] [Google Scholar]
- Factors associated with poor treatment outcome of tuberculosis in Debre Tabor, Northwest Ethiopia. BMC Res Notes. 2018;11:25.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment outcome of new smear positive pulmonary tuberculosis patients in Hamadan, Iran: A registry-based cross-sectional study. Egypt J Chest Dis Tuberc. 2016;65:825-30.
- [CrossRef] [Google Scholar]
- Effect of HIV infection on TB treatment outcomes and time to mortality in two urban hospitals in Ghana-a retrospective cohort study. Pan Afr Med J. 2019;32:206.
- [CrossRef] [PubMed] [Google Scholar]
- Early mortality in new patients on treatment for smear positive pulmonary tuberculosis in Yaounde-Cameroon. 2011. Health Sci Dis. 12:1-7. Avaiable from: https://www.hsd-fmsb.org/index.php/hsd/article/view/21 [Last accessed on 2021 Sep 20]
- [Google Scholar]
- Clinical forms and determinants of different locations of extra-pulmonary tuberculosis in an African country. Indian J Tuberc. 2013;60:107-13.
- [Google Scholar]
- Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:1005-70.
- [CrossRef] [Google Scholar]
- Rapport Annuel 2010 des Activites du Programme National de Lutte Contre le SIDA. Recherche: 2011.
- [Google Scholar]






