Translate this page into:
Factors that influence diagnostic delay among pulmonary tuberculosis patients in Osogbo, Nigeria
*Corresponding author: Olubola Titilope Adegbosin, General Outpatient Department, Military Hospital, Benin, Edo State, Nigeria. olubolaalamu@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Olarewaju SO, Alawode OA, Adegbosin OT, Olaniyan AB, Adeyemo SC. Factors that influence diagnostic delay among pulmonary tuberculosis patients in Osogbo, Nigeria. J Pan Afr Thorac Soc 2023;4(1): 22-30.
Abstract
Objectives:
Delay in the diagnosis of pulmonary tuberculosis (TB) is reportedly common in Nigeria. This results in delayed initiation of treatment and increased spread of the disease. This study assessed diagnostic delay and its influencing factors among pulmonary TB patients who were attending directly observed treatment clinics in Osogbo, Nigeria.
Materials and Methods:
This descriptive cross-sectional study was carried out from August to October 2019 in 10 directly observed treatment clinics in Olorunda Local Government Area, Osogbo, Osun State, Nigeria. Multistage random sampling was used to select 280 registered TB patients. Data were collected using an interviewer-administered semi-structured questionnaire and analyzed with Statistical Package for the Social Sciences version 23.
Results:
Most respondents, that is, 220 (80.3%) were Yoruba, 69 (25.2%) had tertiary education, and 53 (19.3%) had no education at all. Only 135 (49.3%) respondents had good knowledge of pulmonary TB. We found that 157 (57.3%) respondents had patient-related diagnostic delays, while 135 (49.3%) had health system-related delays. Median patient-related delay was 1 month, while median health system-related delay was 3 weeks. There was no significant association between patient-related delay and any sociodemographic characteristic, knowledge about TB, or perceived attitude of health care workers. A significant difference in health-system-related delay was found concerning the attitude of health care workers toward patients (P = 0.043) and patients’ religion (P = 0.030), and level of education (P = 0.001).
Conclusion:
While pulmonary TB is common in the lower socioeconomic class, health workers should be open-minded while evaluating all patients to ensure correct and prompt diagnosis. A good attitude of health workers towards patients is important for preventing diagnostic delays.
Keywords
Determinants
Diagnosis
Diagnostic delay
Tuberculosis
INTRODUCTION
Globally, one out of three persons is infected with Mycobacterium tuberculosis.[1,2] The control of tuberculosis (TB), of which the most common form is pulmonary tuberculosis (PTB), remains a major public health challenge in low-income countries with a high incidence of TB, particularly where human immunodeficiency virus (HIV) infection prevalence is also high.[2] TB is the ninth leading cause of death worldwide. In 2020, there were an estimated 1.3 million TB-related deaths among HIV-negative people and an additional 214,000 deaths among HIV-positive people.[2] There were 9.9 million people infected with TB globally in the same year, equivalent to 127 cases per 100,000 population.[2]
Early diagnosis is a determining factor for the spread of TB as most transmissions occur between the onset of cough and initiation of treatment.[3] Delay in diagnosis and treatment of TB geometrically increases the spread and infectivity of the disease and is associated with a higher risk of mortality.[2] Unfortunately, this has been reported to be common in Nigeria and other countries.[4-6] Delay in the diagnosis of TB has been studied in high-, middle-, and low-income countries and varies significantly, from 63 days in Italy[7] to 88 days in Iran[8] and up to 120 days in Burkina Faso.[9] These delays are attributable both to patients and the health system (including health workers)[4] due to poor health-seeking behavior, inappropriate diagnostic investigations requested by healthcare providers, and limited diagnostic capacities of healthcare facilities.[10,11] Patients experiencing TB symptoms may initially seek relief using self-prescribed medication or by consulting a health-care provider who does not request TB investigations despite repeated visits.[3] In addition, the economic burden of seeking care remains a barrier for many TB patients.[12]
Nigeria is one of the 14 countries with the highest burden of TB, TB/HIV, and multidrug-resistant TB. It is ranked 6th among the 30 high TB burden countries worldwide and first in Africa.[13] In 2019, Osun State had the ninth highest case notification rate for TB among the 36 states in Nigeria. A total of 138,591 new TB cases were notified nationwide in 2020 by the National TB and Leprosy Control Program.[14]
A major issue with TB in Nigeria is the low TB case finding for both adults and children. Many missing TB cases are either not diagnosed or not reported in Nigeria.[14] This problem is, however, not limited to Nigeria. In 2020, globally, there was a decline in TB case notification, with only 5.8 million out of the estimated 9.9 million cases of TB reported.[2]
The control of TB continues to prove difficult due to diagnostic delays, which, in turn, contribute to low case finding. Hence, there is a need to investigate the magnitude and causes of delay in diagnosis among TB patients.[15] Although there have been studies on this subject, only few of them analyzed the factors associated with patient-related and health system-related diagnostic delay separately. This study, therefore, aimed to assess the magnitudes of patient-related and health system-related diagnostic delay and their influencing factors among PTB patients attending directly observed treatment short-course (DOTS) clinics in Osogbo.
MATERIALS AND METHODS
Study design
This was a descriptive and cross-sectional study.
Setting
The study was carried out from August to October 2019 in DOTS clinics in Olorunda and Osogbo Local Government Areas (LGAs) of Osogbo, the capital of Osun State, Nigeria. The city had a population of 324,156 people in 2006, based on the 2006 census,[16] and a projection of 730,642 by 2021.[17]
Participants
Included in the study were patients with PTB between 10 and 50 years of age, who were undergoing treatment at the time of the study, and who were not infected with HIV. We excluded health workers with PTB.
Variables
For questions on knowledge, the responses were either “yes” or “no” (or “correct” or “incorrect”), a correct answer was scored 1 and a wrong answer was scored 0. For questions with three responses (“Yes”, “No,” and “I don’t know”), the correct response was scored 2, “I do not know” was scored 1, while the wrong response was scored 0. Total score and mean score computed were 58 and 35, respectively. Respondents having scores greater than or equal to the mean score were categorized as having good knowledge, while those who scored below the mean score were categorized as having poor knowledge.
For questions on perceived attitude, the responses were “Always,” “Sometimes,” or “Never,” using a Likert-like scale, the responses were scored 3, 2, and 1 in that order for a positive attitude and 1, 2, and 3 in that order for a negative attitude. Maximum and mean scores computed for perceived attitude were 48 and 21.7, respectively. The respondents who had scores below the mean were regarded as having poor perception, while those who scored up to or above the mean were regarded as having good perception.
We categorized diagnostic delay into patient-related delay and health system-related delay. The patient-related delay was measured from the onset of symptoms to the time of presentation in a health facility, while health system-related delay was measured from the time of presentation to the time that diagnosis of PTB was made. The median patient-related delay was 1 month, while the median health system-related delay was 3 weeks. These medians were used as the cutoff values for deciding whether patients had a diagnostic delay or not. We considered patients to have a patient-related delay if they presented at a health facility more than 1 month after the onset of symptoms, and to have a health system-related delay if they were diagnosed more than 3 weeks after presenting.
Data sources/measurement
Study instrument
A semi-structured questionnaire was used to collect information about the patients’ sociodemographic characteristics, knowledge about TB, and diagnostic delay and its likely causes. The questionnaire was divided into two sections. The first section collected data on the sociodemographic characteristics of the respondents; the history of TB, its treatment, and diagnostic delay among respondents; and knowledge of TB among the respondents. The second section focused on the clients’ perception of the technical competence of health-care providers and their attitudes toward patients. The validity of the research instrument was pre-tested among TB patients attending the DOTS clinic in Asubiaro State Hospital.
Data collection
Data were collected using trained research assistants who were final year medical students of Ladoke Akintola University of Technology, Ogbomoso. Training on data collection was done for 2 days, using copies of the questionnaire. The questionnaire was self-administered for literate respondents and interviewer-administered for the illiterate respondents.
Bias
Potential bias in this study was minimized using probability sampling technique. Multistage random sampling was used, as follows.
First stage (selection of LGAs)
One LGA was chosen out of the two LGAs that have DOTS centers in Osogbo (Olorunda and Osogbo LGAs), by simple random sampling (balloting technique).
Second stage (selection of DOTS centers)
Using a simple random technique, 15 out of the 27 DOTS centers that formed the sampling frame in Olorunda LGA were selected.
Third stage (selection of respondents)
Out of the 15 selected DOTS centers, patients were finally recruited based on the proportion allocated to each center through a systematic sampling technique based on sampling fraction.
Sample size
The minimum sample size for the study was calculated using Leslie Fischer’s formula for populations greater than 10,000, which is n=z2pq/d2.[18]
Where,
n = sample size
z = 1.96 (constant)
d = degree of accuracy of study = 0.05
P = the prevalence of the characteristic of interest among the target population, which was taken as 0.79 based on a previous study.[19]
q = 1‒P = 0.21
n = 1.962×0.79×0.21/0.052
n = 254.93≈255
Allowing for a non-response rate of 10%, 280 questionnaires were administered.
Statistical methods
Questionnaires were sorted out to check for errors and omissions at the end of the collection of data. Thereafter, data were analyzed with Statistical Package for the Social Sciences version 23. Frequency distribution tables were generated from variables, while cross-tabulation and test statistics were done where applicable. Univariate analysis was done to determine associations between diagnostic delay and patients’ sociodemographic characteristics, knowledge, and perceived attitude of health workers. Chi-square was used to compare categorical variables and Fisher’s exact test was used when cells had expected values <5. Student’s t-test was used to determine the association between quantitative variables. The level of significance was set at P < 0.05.[14] Multivariate analysis was also done to minimize confounding factors. Variables that had significant association on univariate analysis were included in the multivariate analysis.
Ethics
Ethical approval
Ethical approval for the study was obtained from Osun State Health Research Ethics Committee (approval number OSHREC/PRS/569T/156). The study protocol complied with the ethical standards of the Osun State Health Research Ethics Committee and the Declaration of Helsinki.
Right to decline/withdraw from the study
Respondents were told that participation was voluntary and they would not suffer any consequences if they chose not to participate.
Confidentiality of data
All information gathered were kept confidentially. Participants were identified using serial numbers.
Consent to participate
All participants voluntarily provided informed verbal consent.
RESULTS
The response rate for this study was 98% (274 of 280 respondents). Most of the respondents, that is, 202 (73.7%), were 45 years old or younger, 220 (80.3%) were Yoruba, Muslims constituted 51.1%, 69 (25.2%) had tertiary level of education, and 53 (19.3%) had no education at all. Other sociodemographic findings are shown in Table 1.
| Variable | Frequency, n=274 | Percentage |
|---|---|---|
| Age in categories (years) | ||
| 16‒30 | 92 | 33.6 |
| 31‒45 | 110 | 40.1 |
| 46‒60 | 59 | 21.5 |
| ≥61 | 13 | 4.8 |
| Religion | ||
| Christianity | 122 | 44.5 |
| Islam | 140 | 51.1 |
| Traditional | 12 | 4.4 |
| Ethnicity | ||
| Yoruba | 220 | 80.3 |
| Igbo | 23 | 8.4 |
| Hausa | 29 | 10.6 |
| Others | 2 | 0.8 |
| Marital status | ||
| Single | 83 | 30.3 |
| Married | 168 | 61.3 |
| Divorcee | 11 | 4.0 |
| Separated | 12 | 4.4 |
| Highest educational status | ||
| No formal education | 53 | 19.3 |
| Primary education | 53 | 19.3 |
| Secondary education | 99 | 36.1 |
| Tertiary education | 69 | 25.2 |
| Occupation | ||
| Student | 60 | 21.9 |
| Unemployed | 17 | 6.2 |
| Unskilled laborer | 19 | 6.9 |
| Petty trader | 39 | 14.2 |
| Farmer | 51 | 18.6 |
| Artisan | 64 | 23.4 |
| Professional | 24 | 8.8 |
Only 127 (46.4%) of the respondents knew that PTB is caused by a bacterium. The mode of contracting the disease was rightly identified as coughing/sneezing by 183 (66.8%) respondents. Most of the patients agreed that the disease is curable, that is, 250 (91.2%), and that the best place for treatment is health facilities, that is, 227 (82.8%). The duration of treatment was correctly stated as 6 months by 178 (65.0%) respondents. Table 2 shows the respondents’ answers to questions used to assess knowledge about PTB.
| Variable | Frequency | Percentage |
|---|---|---|
| Etiology | ||
| Bacteria | 127 | 46.4 |
| Smoking | 38 | 13.9 |
| Alcohol intake | 21 | 7.7 |
| Drinking unclean water | 18 | 6.6 |
| Witchcraft | 6 | 2.2 |
| Cough as mode of transmission | 183 | 66.8 |
| Risk factors | ||
| Poverty | 27 | 9.9 |
| Poor nutrition | 29 | 10.6 |
| Overcrowding | 162 | 59.1 |
| Human immunodeficiency virus infection | 9 | 3.3 |
| Symptoms | ||
| Chronic cough | 136 | 49.6 |
| All symptoms (cough, fever, and shortness of breath) | 9 | 3.3 |
| Diagnosis and treatment | ||
| Sputum test as mode of diagnosis | 233 | 85 |
| Disease is curable | 250 | 91.2 |
| Best treated in health facility (DOT) | 227 | 82.8 |
| Treatment period: 6 months for Regimen 1 | 178 | 65.0 |
| Treatment period: 12 months for Regimen 2 | 1 | 0.4 |
| Prevention | ||
| Improved ventilation | 53 | 19.3 |
| Proper sputum disposal | 24 | 8.8 |
| Avoidance of overcrowding | 59 | 21.5 |
| Avoidance of direct coughing | 75 | 27.4 |
DOT: Directly observed therapy
The mean PTB knowledge score was 35; 135 (49.3%) respondents had scores ≥35 and were classified as having good knowledge of PTB, while 139 (50.7%) respondents had scores <35 and were classified as having poor knowledge [Figure 1].

- Summarized knowledge on tuberculosis among respondents.
Respondents’ answers to questions that were used to assess their perception of health workers’ attitudes are shown in [Table 3]. The mean score for the perception of health workers’ attitudes was 22; 111 (40.5%) respondents had scores ≥22 and were classified as having good perception of health workers’ attitudes, while 163 (59.5%) respondents had scores <22 and were classified as having poor perception [Figure 2].
| Variable | Always, n(%) | Sometimes, n(%) | Never, n(%) |
|---|---|---|---|
| Health workers are active listeners | 172 (62.8) | 96 (35) | 6 (2.2) |
| Health workers encourage clients and family members | 208 (75.9) | 64 (23.4) | 2 (7) |
| Health workers give patients full attention during consultation | 211 (77) | 60 (21.8) | 3 (1.1) |
| Health workers care about what patient is saying | 179 (65.3) | 92 (33.6) | 3 (1.1) |
| Health workers allow patient to be active in decision-making | 152 (55.5) | 112 (40.9) | 19 (3.6) |
| Health care workers communicate well in simple language | 196 (71.5) | 75 (27.4) | 3 (1.1) |
| Health care workers demonstrate excellent control services | 193 (70.4) | 76 (27.7) | 5 (1.8) |
| Health care workers have sincere interest in their patients | 203 (74.1) | 68 (24.8) | 3 (1.1) |
| Health care workers care about you as a person | 196 (71.5) | 73 (26.6) | 5 (1.8) |
| Health care workers show interest in all patients | 194 (70.8) | 73 (26.6) | 7 (2.6) |
| Health care workers are compassionate | 207 (75.5) | 64 (23.4) | 3 (1.1) |
| Health care workers practice confidentiality | 215 (78.5) | 54 (19.7) | 5 (1.8) |
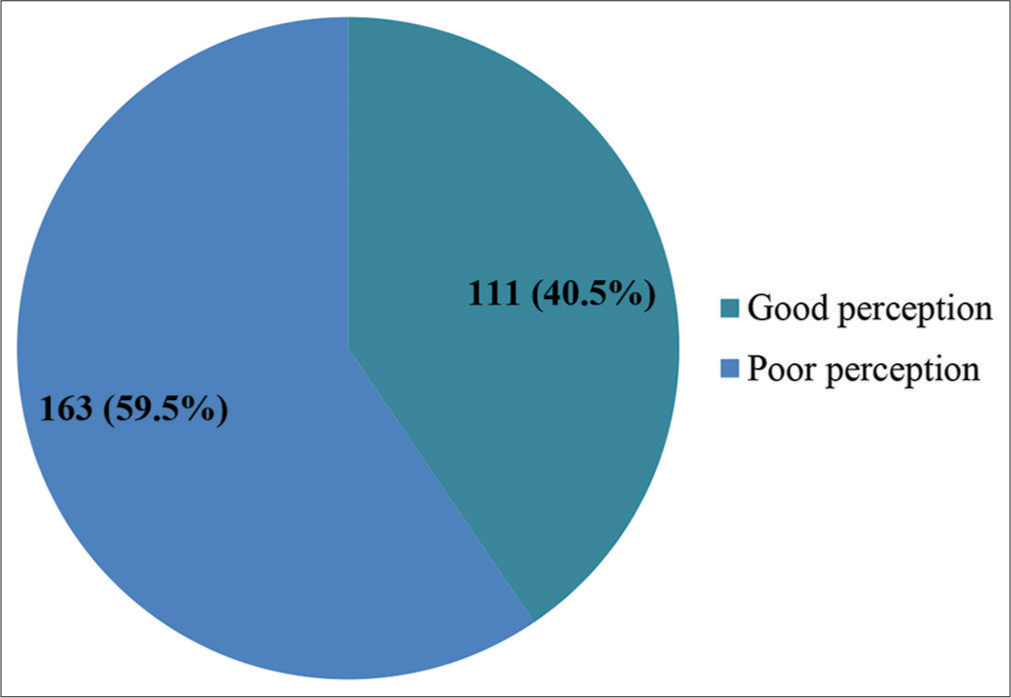
- Categorized perception of health care workers’ attitudes among respondents.
Based on the criteria set for patient-related and health system-related diagnostic delay, 157 (57.3%) patients had a patient-related delay, while 117 (42.7%) did not have a patient-related delay [Figure 3]. About 135 (49.3%) respondents had health system-related delays, while 139 (50.7%) did not have health system-related delays [Figure 4]. The median patient-related delay was 1 month, while the median health system-related delay was 3 weeks.
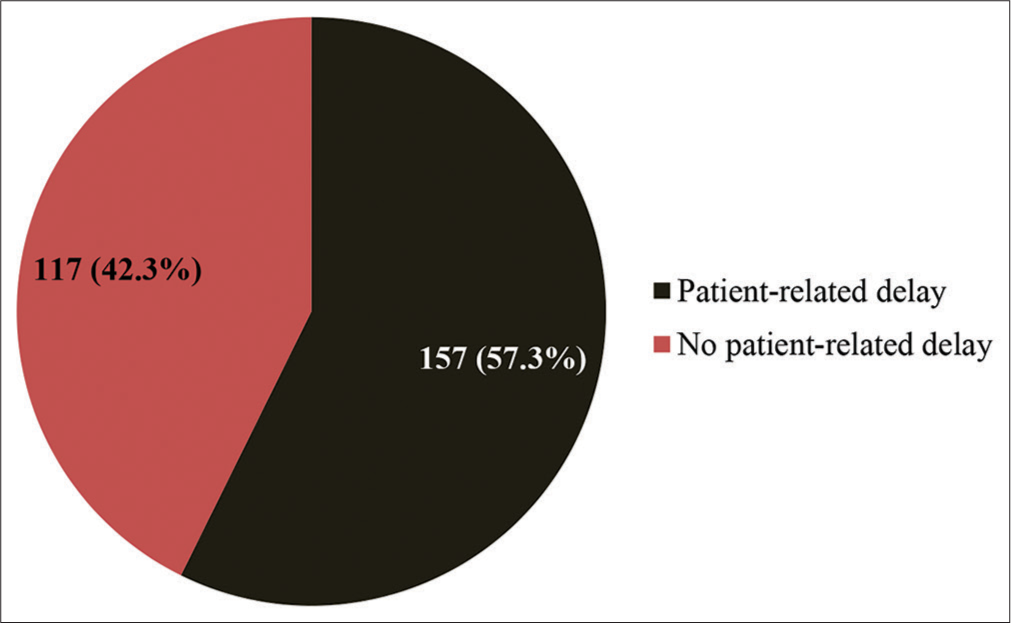
- Categorized patient-related delay among respondents.
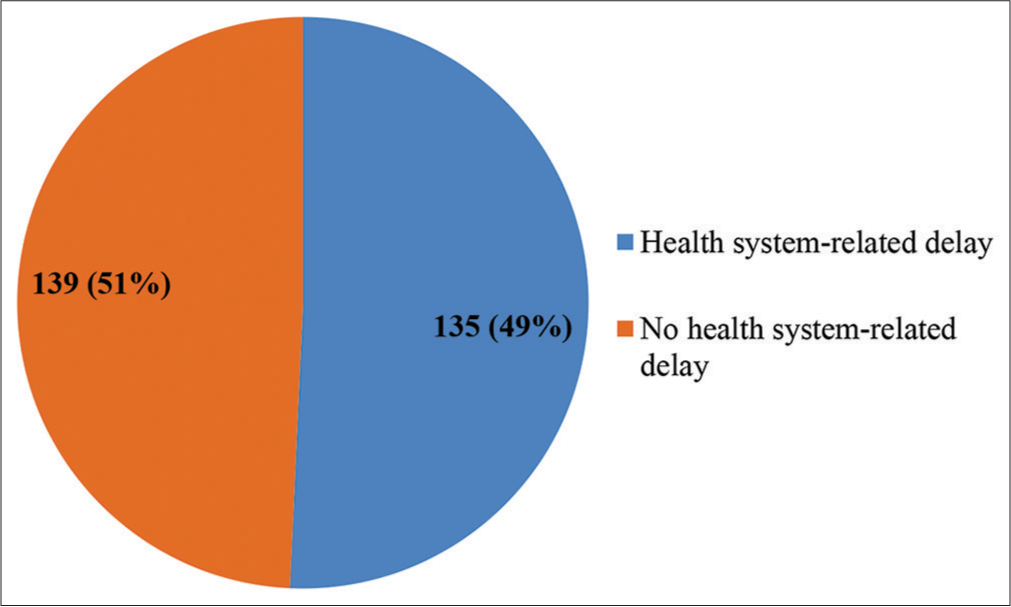
- Categorized health system-related delay among respondents.
We analyzed the relationship between sociodemographic characteristics and patient-related diagnostic delay. Univariate analysis showed no significant relationship between patient-related diagnostic delay and the sociodemographic characteristics (P > 0.05). Patients’ TB knowledge score and perception of health care workers’ attitudes also had no significant relationship with patient-related diagnostic delay. On multivariate analysis, none of the variables showed a statistically significant association with patient-related diagnostic delay (P > 0.05) [Table 4].
| Variable | No patient-related diagnostic delay, n(%) | Patient-related diagnostic delay, n(%) | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| X2 | P-value | OR | CI | |||
| Age in categories (years) | 2.06 | 0.561 | ||||
| 16–30 | 38 (41.3) | 54 (58.7) | 2.27 | 0.69–2.87 | ||
| 31–45 | 47 (42.7) | 63 (57.3) | 2.15 | 0.65–6.97 | ||
| 46–60 | 24 (40.7) | 35 (59.3) | 2.33 | 0.68–8.00 | ||
| ≥61 | 8 (61.5) | 5 (38.5) | 1 | |||
| Ethnicity | 0 | 0.97 | ||||
| Yoruba | 94 (42.7) | 126 (57.3) | 0.99 | 0.5–5.83 | ||
| Others | 23 (42.6) | 31 (57.4) | 1 | |||
| Religion | 2.38 | 0.34 | ||||
| Christianity | 46 (37.7) | 76 (62.3) | 1.18 | 0.35–3.94 | ||
| Islam | 66 (47.1) | 74 (52.9) | 0.81 | 0.24–2.65 | ||
| Traditional | 5 (41.7) | 7 (58.3) | 1 | |||
| Highest educational status | 0.26 | 0.97 | ||||
| None | 24 (45.3) | 29 (54.7) | 0.93 | 0.45–1.91 | ||
| Primary | 22 (41.5) | 31 (58.3) | 1.08 | 0.53–2.23 | ||
| Secondary | 41 (41.4) | 58 (58.6) | 1.09 | 0.58–2.03 | ||
| Tertiary | 30 (43.5) | 39 (56.5) | 1 | |||
| Marital status | 0.47 | 0.93 | ||||
| Single | 36 (43.4) | 47 (56.6) | 1.31 | 0.38–4.39 | ||
| Married | 71 (42.3) | 97 (57.7) | 1.37 | 0.43–4.41 | ||
| Divorcee | 4 (36.4) | 7 (63.8) | 0.33 | 0.33–9.29 | ||
| Separated | 6 (50) | 6 (50) | 1 | |||
| Categorized perception | 0.26 | 0.56 | ||||
| Poor perception | 72 (44.2) | 91 (55.8) | 0.86 | 0.52–1.41 | ||
| Good perception | 45 (40.5) | 66 (59.5) | ||||
| Categorized knowledge | 1.9 | 0.17 | ||||
| Poor knowledge | 65 (46.8) | 74 (53.2) | 0.72 | 0.44–1.15 | ||
| Good knowledge | 52 (38.5) | 83 (61.5) | 1 | |||
OR: Odds ratio, CI: Confidence interval
Univariate analysis showed significant relationship between health system-related diagnostic delay and perceived attitude of health care workers to patients (P = 0.043), religion (P = 0.030), and level of education (P = 0.001) [Table 5]. On multivariate analysis, only religion (P = 0.04) and educational status (P = 0.001) showed statistically significant association with health system-related diagnostic delay [Table 5].
| Variable | No health system-related delay, n(%) | Health system-related delay, n(%) | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| X2 | P-value | OR | CI | |||
| Age in categories (years) | 2.14 | 0.55 | ||||
| 16–30 | 44 (47.8) | 48 (52.2) | 2.27 | 0.69–7.48 | ||
| 31–45 | 56 (50.9) | 54 (49.1) | 2.15 | 0.66–6.98 | ||
| 46–60 | 29 (49.2) | 30 (50.8) | 2.33 | 0.68–8.00 | ||
| ≥61 | 9 (59.2) | 4 (30.8) | 1 | |||
| Ethnicity | 0.00 | 1.0 | ||||
| Yoruba | 114 (51.8) | 106 (48.2) | 0.82 | 1.45–1.48 | ||
| Others | 25 (46.3) | 29 (53.7) | 1 | |||
| Religion | 6.45 | 0.04* | ||||
| Christianity | 51 (41.8) | 71 (58.2) | 1.18 | 0.35–3.94 | ||
| Islam | 80 (57.1) | 60 (42.9) | 0.80 | 0.24–2.65 | ||
| Traditional | 7 (58.3) | 5 (41.7) | 1 | |||
| Highest educational status | 16.4 | 0.001* | ||||
| None | 39 (73.6) | 14 (26.4) | 0.92 | 0.45–1.91 | ||
| Primary | 28 (52.8) | 25 (47.2) | 1.08 | 0.53– 2.24 | ||
| Secondary | 44 (44.4) | 55 (55.6) | 1.08 | 0.58–2.03 | ||
| Tertiary | 27 (39.1) | 42 (60.9) | 1 | |||
| Marital status | 0.4 | 0.94 | ||||
| Single | 44 (53) | 39 (47) | 1.31 | 0.38–4.39 | ||
| Married | 83 (49.4) | 85 (50.6) | 1.37 | 0.42–4.41 | ||
| Divorced | 5 (45.5) | 6 (54.5) | 1.75 | 0.33–9.29 | ||
| Separated | 6 (50) | 6 (50) | 1 | |||
| Categorized perception | 3.51 | 0.061 | ||||
| Poor perception | 75 (46.0) | 88 (54.0) | 1.2 | 0.74–1.97 | ||
| Good perception | 64 (57.7) | 47 (42.3) | 1 | |||
| Categorized knowledge | 0.52 | 0.47 | ||||
| Poor knowledge | 73 (52.5) | 66 (47.5) | 0.713 | 0.44–1.15 | ||
| Good knowledge | 65 (48.1) | 70 (51.9) | 1 | |||
DISCUSSION
This study assessed the magnitude of diagnostic delay among PTB patients who were attending DOTS clinics in Osogbo and identified factors that were responsible for the diagnostic delay.
More than half of the respondents in this study had poor knowledge about the etiology, spread, and prevention of PTB. Although the level of knowledge was not associated with diagnostic delay, it is still a matter of concern that these patients did not have a good understanding of the disease, they were being treated for. This may reflect poor health education and counseling on the part of the health-care providers.
The median patient-related delay was 1 month in our study. This is similar to the finding in Malaysia[20] and Ghana,[21] while a shorter duration of 1 week was observed in Auckland, New Zealand.[22] This difference may be because New Zealand is a more developed country than Nigeria, Malaysia, or Ghana.
It was discovered that almost half of the respondents were delayed in presenting to a health facility. This result is discouraging, suggests poor health-seeking behavior, and is significant for public health. Diagnostic delay translates into a delay in the initiation of treatment. These untreated patients come in contact with other people in the community, who are then at risk of contracting the disease. Surprisingly, our analysis showed no significant association between any of the sociodemographic characteristics and patient-related diagnostic delay. There was also no significant association with either TB knowledge score or perceived attitude of health care workers. It is expected that the time of presentation in a health facility after the onset of an illness should be influenced by such factors as educational status, occupation, age, and knowledge about the disease. For example, professionals and people with high level of education are expected to present early at health facilities because they are expected to have good health-seeking behavior based on their exposure. People who are knowledgeable about a disease are expected to seek medical care earlier than those who do not. Furthermore, younger people, being usually more physically and financially independent than the elderly, are expected to seek medical care earlier. Our study results, however, failed to show any association between patient-related diagnostic delay and any of these factors. In contrast to our findings, significant associations between patient-related delay and knowledge about TB, religion, level of education, and marital status were found in a study done in Kerala, India, although it was cross-sectional descriptive in nature and done only among 302 TB patients during the intensive phase of treatment.[23] In that study also, older patients were more likely to have a patient-related delay.[23] Likewise, another study in Zambia also found an association between level of education and patient-related delay.[6]
The median duration for health system-related delay was 3 weeks in our study, which is shorter than the 7 weeks reported in a study in New Zealand.[22] This may be due to a higher index of suspicion for TB in Nigeria, being a high TB burden country, unlike New Zealand. There was a difference in health system-related delay concerning religion, level of education, and perceived attitude of health workers to patients. Health system-related delay was less common among people with the low level of education. This may reflect that health workers consider TB as primarily a disease of people with low socioeconomic status and, therefore, have a higher index of suspicion for it among such people than among people of higher socioeconomic status. In a similar finding in China, it was found that people with the highest income were at risk of having diagnostic delays.[20]
In contrast, a study done in Tanzania found no difference in diagnostic delay concerning income or educational status, while another study in Tunisia found that health system-related delay was associated with older age.[24,25]
Religion was found to be associated with health system-related delay, with Muslims having lower odds and Christians having higher odds of delay when compared with traditional worshippers. This may reflect the high level of dependence on faith healing and herbal remedies among Christians and traditional worshippers, respectively. In contrast, a qualitative study done in a rural district of Mwanza, Tanzania, found that Christians had shorter diagnostic delay than others (people who had no religion or who believed in bewitchment).[26] The general perception of the respondents in that study was that God, Allah, and witchcraft may all be responsible for diseases, and that while diseases caused by God or Allah can be treated in the hospital, only traditional healers can treat those caused by witchcraft. These findings underscore the important role that religious/cultural beliefs play in modifying health-seeking behavior. They also imply that religious and cultural leaders are important agents for effecting change in health-related practices of people in a community.
Health system-related delay in our study was also commoner in facilities where patients reported poor attitude of health workers toward them. This is not surprising, as a poor attitude toward patients will result in poor health worker-patient interaction and poor service delivery.
The findings of this study re-emphasize the importance of incorporating patient education into patient management, as good awareness equips patients to cooperate with healthcare providers for the management of their disease conditions. This will translate into good medication compliance, regular clinic attendance, and prevention of the spread of communicable diseases. Moreover, patients with good knowledge of their diseases will be able to pass on such knowledge to their families and communities, thereby increasing public awareness and promoting public health. This education should not only focus on people of low socioeconomic status but well-educated and wealthy people also need to be educated on good health-seeking behavior.[27]
Furthermore, health workers should be open-minded when evaluating patients. While some diseases are common among certain sociodemographic groups, they should not be excluded as possibilities in other groups, especially when they are of public health importance, and symptoms suggestive of them are present. Health care workers also need to maintain a good attitude toward patients and be committed to productive relationships with patients and effective service delivery at all times. This will facilitate comprehensive patient evaluation, prompt diagnosis, and effective treatment.
The major limitation of our study is the lack of complementary qualitative insight. Data collection through interviews or focus group discussions with patients and health workers would have provided more robust answers to the question of factors that cause a diagnostic delay in patients with PTB. The future studies on this subject will do well to utilize both quantitative and qualitative analyses.
CONCLUSION
This study found inadequate knowledge about PTB among patients who were being treated for the disease. There was also significant delay in diagnosis due to both patient-related and health system-related factors. These findings call for intensified public health efforts in health education of communities about PTB and continuous training of health workers on the importance and means of early diagnosis. Future mixed-method research on diagnostic delay in PTB will provide further insight on the subject.
Acknowledgment
The authors thank the research assistants who helped with data collection, and the health care workers and study participants for their cooperation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Barriers in the management of tuberculosis in Rawalpindi, Pakistan: A qualitative study. Tanaffos. 2013;12:28-34.
- [Google Scholar]
- Global Tuberculosis Report 2021. 2021. Geneva: World Health Organization; Available from: http://www.who.int/tb/publications/global_report/gtbr2021_main_text.pdf [Last accessed on 2021 Nov 27]
- [Google Scholar]
- A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15.
- [CrossRef] [PubMed] [Google Scholar]
- Hospital prevalence of pulmonary tuberculosis and co-infection with human immunodeficiency virus in Ilorin: A review of nine years (1991-1999) West Afr J Med. 2002;21:24-7.
- [Google Scholar]
- Care-seeking behavioural patterns, awareness and diagnostic processes in patients with smear-and culture-positive pulmonary tuberculosis in Lagos, Nigeria. Trans R Soc Trop Med Hyg. 2002;96:614-6.
- [CrossRef] [Google Scholar]
- Barriers to tuberculosis control in urban Zambia: The economic impact and burden on patients prior to diagnosis. Int J Tuberc Lung Dis. 1998;2:811-7.
- [Google Scholar]
- Delay in the treatment of pulmonary TB in a changing demographic scenario. Int J Tuberc Lung Dis. 2006;10:305-9.
- [Google Scholar]
- Diagnostic and Treatment Delay in Tuberculosis. 2006. Geneva: WHO World Health Organization; Available from: http://www.emro.who.int/dsaf/dsa710.pdf [Last accessed on 2021 Nov 27]
- [Google Scholar]
- Treatment seeking behaviour of smear-positive tuberculosis patients diagnosed in Burkina Faso. Int J Tuberc Lung Dis. 2006;10:184-7.
- [Google Scholar]
- Regional Office for South-East Asia. Tuberculosis Control in the South-East Asia Region. 2011. The Regional Report. Geneva: World Health Organization; Available from: https://apps.who.int/iris/handle/10665/206066 [Last accessed on 2021 Nov 27]
- [Google Scholar]
- Does intensified case finding increase tuberculosis case notification among children in resource-poor settings? A report from Nigeria. Int J Mycobacteriol. 2016;5:44-50.
- [CrossRef] [PubMed] [Google Scholar]
- Delays in diagnosis and treatment of pulmonary tuberculosis in Wakiso and Mukono districts, Uganda. BMC Public Health. 2014;14:586.
- [CrossRef] [PubMed] [Google Scholar]
- TB in Nigeria Funding, Children, Diagnosing TB, HIV/TB. Available from: https://tbfacts.org/tb-nigeria [Last accessed on 2021 Nov 27]
- [Google Scholar]
- Federal Ministry of Health, Nigeria 2020 Annual TB Report. Nigeria: Federal Ministry of Health; 2020.
- [Google Scholar]
- Patterns of delays amongst pulmonary tuberculosis patients in Lagos, Nigeria. BMC Public Health. 2004;4:18.
- [CrossRef] [PubMed] [Google Scholar]
- Legal Notice on Publication of 2006 Census Final Results. Federal Republic of Nigeria Official Gazette. 2009;96:37-8.
- [Google Scholar]
- Oshogbo, Nigeria Population. Available from: https://populationstat.com/nigeria/oshogbo [Last accessed on 2021 Nov 27]
- [Google Scholar]
- Mycobacteria In: Sherris Medical Microbiology: An Introduction to Infectious Diseases (4th ed). New York: McGraw-Hill; 2004. p. :439.
- [Google Scholar]
- Diagnostic delay among pulmonary tuberculosis patients in Sarawak, Malaysia: A cross-sectional study. Rural Remote Health. 2007;7:667.
- [CrossRef] [Google Scholar]
- Pulmonary tuberculosis: Diagnostic delay in Ghanaian adults. Int J Tuberc Lung Dis. 1998;2:635-40.
- [Google Scholar]
- Diagnostic delay and associated factors among patients with pulmonary tuberculosis in Kerala. J Family Med Prim Care. 2017;6:643-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic delay and associated factors among patients with pulmonary tuberculosis in Dar es Salaam, Tanzania. Infect Dis Poverty. 2017;6:64.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary tuberculosis: Diagnostic delay in Tunisia. Med Mal Infect. 2016;46:79-86.
- [CrossRef] [PubMed] [Google Scholar]
- Factors underlying diagnostic delay in tuberculosis patients in a rural area in Tanzania: A qualitative approach. Infection. 2010;38:433-46.
- [CrossRef] [PubMed] [Google Scholar]
- Literacy and health seeking behaviors among patients in Benue and Cross River States of Nigeria. J Contemp Res. 2010;7:58-63.
- [CrossRef] [Google Scholar]






