Translate this page into:
The accuracy of a mobile phone application (Wulira app) compared to standard audiometry in assessing hearing loss among patients on treatment for multidrug-resistant tuberculosis in Uganda
*Corresponding author: Charles Batte, Department of Medicine, Lung Institute, Makerere University College of Health Sciences, Kampala, Uganda batchaux@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Batte C, Olayanju T, Mukisa J, Namusobya MS, Alenoghena I, Sulaiman L, et al. The accuracy of a mobile phone application (Wulira app) compared to standard audiometry in assessing hearing loss among patients on treatment for multidrug-resistant tuberculosis in Uganda. J Pan Afr Thorac Soc 2020;1(1):20-5.
Abstract
Objectives:
Our aim was to validate the “Wulira App” a mHealth application against gold standard audiometry as a pragmatic audiometry solution for under-served and vulnerable groups of patients at risk of hearing loss. The specific objectives were as follows:
To compare hearing thresholds determined using the Wulira app to standard pure tone audiometry among patients on MDR-TB treatment. To determine the correlation between the measured hearing loss with the Wulira app and standard audiometry with patient reported hearing loss. To determine the proportion of patients on MDR-TB treatment that experience hearing loss?
Materials and Methods:
We consecutively recruited patients ≥18 years old and receiving kanamycin in their treatment regimen between February and June 2019 for this study. Clinical and demographic data were obtained from each participant and documented in a secure database. Participants had hearing assessment performed once at enrolment with paired standard audiometry and the Wulira mobile phone app in a soundproof room.
Results:
A total of 120 MDR-TB patients with a mean age of 34.0 (±9.6) years were recruited for this study and 69 (57.5%) were male. When compared to pure tone audiometry, the Wulira app was able to correctly detect 91.4% hearing loss in right ear and 88.4% in the left ear. The specificity of the Wulira app was equally high, reaching 93.2% in the right ear and 91.5% in the left ear.
Conclusion:
The Wulira app may be a useful alternative home-based tool for hearing assessment in MDR-TB patients, essentially for early detection of hearing loss following commencement of second-line injectable drugs.
Keywords
mHealth
Mobile audiometry
Hearing loss
Ototoxicity
INTRODUCTION
Tuberculosis (TB) remains a major public health threat. Multidrug-resistant strains of Mycobacteria tuberculosis are increasingly common, compromising the recent gains in global TB control.[1] Operationally, second-line injectable aminoglycosides form the backbone of multidrug-resistant TB (MDR-TB) treatment regimens.[2,3] Ototoxicity and hearing loss are a common side effect with prevalence between 18% and 62% among MDR-TB patients.[4-6] Regular hearing assessment is required to monitor for and prevent disabling hearing loss, but limited availability of audiologists and audiometric equipment impedes the provision of these services in sub-Saharan Africa (SSA).[7] Our aim was to validate the “Wulira App” a mHealth application against gold standard, pure tone audiometry (PTA) as a pragmatic audiometry solution for this underserved and vulnerable group of patients.
PTA is the gold standard assessment tool to monitor hearing loss, but this technique is expensive and requires a trained operator in a controlled environment for use.[8] This is not practicable in high MDR-TB prevalence resource poor countries. Recently, a number of smartphone applications have been developed for hearing assessment, they include SPLnFFT, SoundMeter, NoISee, and uHear among others. These aim to increase accessibility to hearing assessment, although there is a paucity of robust validation data for use of these tools;[9] this limits effective implementation in health-care settings. Furthermore, the tone-based mobile apps have variable accuracy depending on headphone and phone type used.[10]
In this study, we examined the “Wulira app,” a locally designed user-friendly mobile smartphone audiometry application[11] to assess hearing loss among MDR-TB patients receiving an aminoglycoside-based treatment regimen in a clinical setting. This tool was compared against PTA, the gold standard for hearing assessment by comparing measured hearing threshold under the same conditions. If accuracy was demonstrated, this pragmatic and accessible tool could improve safe aminoglycoside treatment delivery and minimize hearing loss for MDR-TB patients in low-income settings.
MATERIALS AND METHODS
Study design
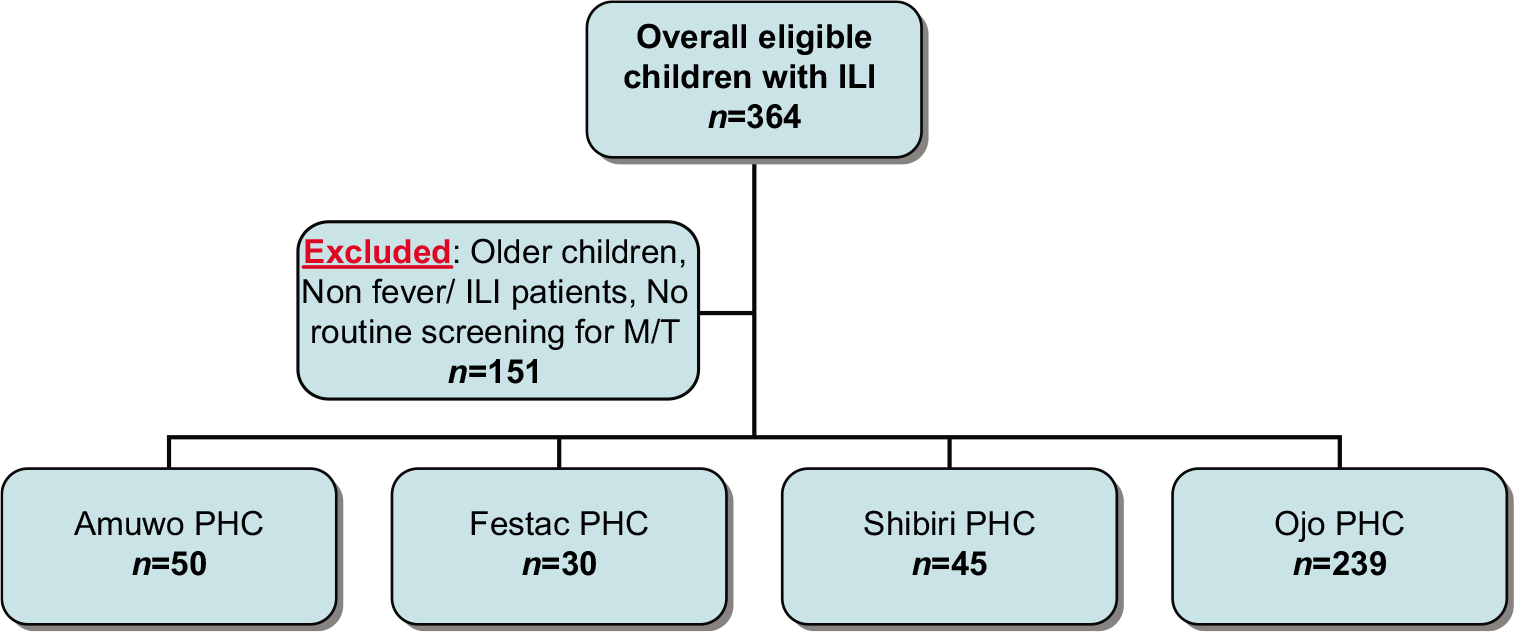
This was a cross-sectional study involving MDR-TB patients receiving aminoglycoside-based treatment at Mulago National Referral Hospital MDR-TB Clinic, Kampala, Uganda. The hospital serves as the national referral for MDRTB treatment in Uganda and has PTA facilities run by a trained audiologist. We consecutively recruited patients ≥18 years old and receiving kanamycin in their treatment regimen between February and June 2019 for this study. We excluded patients with a history of occupational hearing loss, inability to use PTA, and those who lacked capacity. No formal power calculation was performed for this exploratory study. This being a feasibility study, the sample size for this study has been determined on pragmatic principles considering the number of cases we expected to see at the center during the study period. At the time, an average of 120 patients was enrolled into MDR TB care. Mulago Hospital Research and Ethics Committee (MREC 1539) and Uganda National Council of Science and Technology (SS #4913) approved the study protocol.
Description of the hearing assessment approaches
Wulira app
The Wulira app is an android operating system smartphone mobile app that is freely downloadable at https://wuliraapp.com/about-us/ and Google Play store.[11] It produces sounds of frequencies 125–8000 Hz and amplitudes between 0 and 100 dB. A patient may use standard headphones to self-test themselves. The entire testing process is automated, requiring the patient to only click on the response button when a test sound is heard. The app records each of the patient’s responses and plots an audiogram for the test session as shown in [Figure 1]. It then uses an inbuilt algorithm to compute the average hearing threshold for the patient and generates a diagnosis based on the WHO classification of hearing loss. The results on the phone can also be shared with the audiologist or an ear, nose, and throat specialist for further advice and management. All data collected are protected and stored centrally and can be made available for the subsequent testing sessions as a baseline for comparing future tests to note any changes in hearing thresholds of the patient.
- Screenshot of the Wulira App interface showing the testing dashboard and an audiogram.
PTA
PTA is a procedure carried to test hearing of an individual in a soundproof room using headphones. An audiometer operated by the skilled radiologist delivers sound intensities to each ear separately and the frequency at which an individual hears is documented. The PTA is calibrated daily before commencing of hearing assessments for patients by the audiologist to minimize errors.
Study procedure
Clinical and demographic data were obtained from each participant after explaining the study procedures and obtaining written informed consent. All data were entered into a secure database. Before the start of the study, one radiologist (with 1 year experience in audiometry) underwent training. The training included how to use the Wulira app, PTA, and completion of the study data collection tools. She consistently performed hearing assessment once for all participants at enrolment with PTA and the Wulira mobile phone app in a soundproof room. The hearing threshold of 25 dB was taken as normal while threshold above 25 dB was taken as abnormal.[12] The assessment was done at 500 Hz, 1000 Hz, 2000 Hz, 3000 Hz, and 4000 Hz, with both ears tested one after the other as recommended by the World Health Organization.[13] The results obtained from both instruments were recorded, outputs from PTA were used to determine the presence of hearing loss, and outputs from Wulira app were compared with the PTA readings. The degree of agreement of hearing threshold measured by the two instruments was also determined. Participants were asked to make a subjective report of their own hearing ability and this was compared with measurements from both instruments.
Statistical analysis
Categorical variables were summarized using frequencies and proportions, while continuous variables were summarized using means and standard deviations. The sensitivity, specificity, and positive predictive value (PPV) and negative predictive values (NPV) of the Wulira app were calculated for both ears at the different frequencies with reference to PTA. The area under the receiver operating characteristic curves, using the Wulira app for both ears at the different frequencies, was also determined with respect to the PTA. Statistical analysis was performed using STATA 15.0 (StataCorp, Texas, USA).
RESULTS
Demographics and clinical characteristics
We recruited 120 MDR-TB patients with mean age of 34.0 (±9.6) years of age. Sixty-nine (57.5%) of the participants were male. No patients had a prior formal diagnosis of hearing impairment, but 12 patients (10.0%) reported subjective poor hearing on direct questioning. Thirty-six (30.0%) of the patients were still receiving the injectable aminoglycosides at the time of recruitment. Of the remainder, 34 (28.3%) had been on MDR-TB treatment for more than 1 year, 32 (26.7%) patients for seven to 12 months, and 18 (15.0%) patients for 6 months or less [injectable component of treatment completed; Table 1].
| Characteristic | Frequency (percentage) |
|---|---|
| Age (Mean ± SD) years | 33.98 ± 9.59 |
| Patient reported hearing loss in the right ear | 17 (14.2) |
| Patient reported hearing loss in the left ear | 14 (11.7) |
| Patient currently receiving injectable/ototoxic drug | 36 (30.0) |
| Patients reported perception of hearing quality | |
| Poor | 12 (10.0) |
| Good | 101 (84.2) |
| Very good | 7 (5.8) |
Frequency of hearing loss
The frequency of hearing loss among participants, as measured by the PTA in the right ear, was 26.7% at 500 Hz, 19.2% at 1000 Hz, 17.5% at 2000 Hz, and 30.0% at 4000 Hz. In the left ear, the prevalence of hearing loss was 25.0% at 500 Hz, 17.5% at 1000 Hz, 16.7% at 2000 Hz, and 35.0% at 4000Hz.
Wulira app detection of hearing loss
When compared to PTA in terms of sensitivity, the Wulira app was able to correctly detect 91.4% hearing loss in right ear and 88.4% in the left ear. The specificity of the Wulira app was equally high, reaching 93.2% in the right ear and 91.5% in the left ear. The PPV and the NPV are reported in [Table 2].
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | |
| Normal (25 dB) | 91.4 | 88.4 | 92.3 | 91.5 | 96.9 | 96.4 | 80.6 | 75.4 |
| Mild (40 dB) | 55.1 | 51.7 | 91.8 | 90.2 | 49.6 | 45.8 | 93.3 | 92.1 |
| Moderate (50 dB) | 33.3 | 33.3 | 96.3 | 95.7 | 23.1 | 18.6 | 97.8 | 98 |
| Severe (70 dB) | 31.7 | 45.9 | 97.9 | 97.0 | 43.3 | 41.5 | 96.5 | 97.5 |
PPV: Positive predictive value, NPV: Negative predictive value
Performance characteristics of Wulira app
The ability of the Wulira app to detect with accuracy, the hearing threshold in all participants, when compared to the PTA (sensitivity), was 81.3% in the right ear and 76.6% in the left ear. The specificity, PPV, and the NPV are reported in [Table 3]. The extent of accuracy achieved by the Wulira app when compared to the PTA at different frequencies is shown as area under the receiver operating curve in [Figure 2].
| Frequency (Hz) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | |
| 500 | 81.3 | 76.7 | 85.2 | 75.6 | 66.7 | 51.1 | 92.6 | 90.7 |
| 1000 | 87.0 | 90.5 | 95.9 | 96.0 | 83.3 | 82.6 | 96.9 | 97.9 |
| 2000 | 95.2 | 95.0 | 98.0 | 98.0 | 90.9 | 90.5 | 99.0 | 99.0 |
| 4000 | 100 | 88.1 | 91.7 | 91.0 | 83.7 | 84.1 | 100.0 | 93.4 |
PPV: Positive predictive value, NPV: Negative predictive value

- The receiver operating curves comparing hearing threshold detection by Wulira app to the pure tone audiometry at different WHO recommended frequencies 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz.
DISCUSSION
In this study, we found a high prevalence of hearing loss among MDR-TB patients receiving aminoglycoside-based treatment regimen. Wulira app demonstrated high sensitivity and specificity at detecting hearing loss against gold standard PTA. These data demonstrate that the Wulira app could potentially be deployed as a hearing screening tool in low-income settings where access to PTA is limited. Further work is required to confirm Wulira app sensitivity to detect hearing loss in a longitudinal study and determine the most context appropriate headsets for use.
Hearing loss is a major sequela of aminoglycoside-containing regimen in MDR-TB patients.[14,15] Several studies have reported varying prevalence rates.[16,17] Our study showed a prevalence, measured by PTA ranging between 16.0% and 35.0% in the right and left ears across frequencies 500, 1000, 2000, and 4000 HZ and involved mainly the higher frequency (4000 Hz). This is similar to the findings of Duggal and Sarkar who reported 18.75% sensorineural hearing loss involving mainly the higher frequencies.[18] The rationale being that sounds of high frequencies localize near the base of the cochlea which is more susceptible to injury than the apical region.[19] This “cochleotopic” gradient of susceptibility is expressed as high-frequency hearing loss, which extends to include progressively lower frequencies with more extensive cochlear damage.[20] HIV coinfection usually contributes to higher prevalence of hearing loss in affected patients.[17,21]
Calibration of audiometric devices is a key consideration to ensure test accuracy, however, no standardized calibration procedure currently exists for performing tests on smartphone devices coupled with non-audiometric headphones.[22] Several recent studies have investigated calibration methods, but further research evidence is necessary to establish their applications.[23,24] The Wulira app, tested alongside the PTA, demonstrated strong sensitivity in detecting hearing threshold and identification of hearing loss in MDR-TB patients on aminoglycoside-based regimen. This finding is similar to results from other available mobile apps included in a review article.[9] Wulira app has the advantage over those reported tools, of being tested simultaneously with the gold standard PTA in a controlled environment, under the watch of trained audiologist, and these could attest for its favorable applicability for patients’ use.
The major strength of this study was that the hearing assessment was done using the app and the PTA at the same time on every patient to minimize the effect of potential biases. There are, however, some limitations. Poor sound attenuation provided by commercially available ear buds might constitute a challenge for home use where soundproof environment is not guaranteed, this may be minimized by making headphones with greater attenuation of ambient noise available at subsidized rate for the patients. Furthermore, this was a cross-sectional study where only a single reading was done in comparison with the gold standard, essentially to take a snapshot of the accuracy of the mobile app, a longitudinal study with follow-up comparison would provide valuable information on the performance of the app. Our study participants may have other underlying causes of hearing loss such as diuretics, quinine, cytotoxic agents, and chronic environmental noise exposure which negatively influence hearing assessments.
CONCLUSION
The Wulira app may be a useful alternative home-based tool for hearing assessment in MDR-TB patients, essentially for early screening of hearing loss following commencement of second-line injectable drugs. Although the sensitivity reduces when applied out of the normal hearing range, its applicability at 25 decibels is sufficient for objective assessment. When monitoring for ototoxicity, the aim is to detect hearing loss before it affects patients’ capacity for effective communication. Wulira app has potential for clinical settings, especially in resource scarce settings, where the use of PTA is not feasible. This research supports further exploration and validation of the Wulira app as a potential solution for accessible hearing assessment in resource-constrained settings.
Acknowledgments
We acknowledge the support of the National Tuberculosis and Leprosy Program (NTLP) and the staff of Ward 5 and 6 at Mulago National Referral Hospital.
Authors’ contributions
CB conceived the study idea, developed the Wulira app, developed study protocol, and obtained required IRB approvals and supervised the implementation of the study. TO contributed to the study design, drafted manuscript. JM conducted data analysis, contributed to interpretation of the data. NMS supervised patient recruitment, data collection, and cleaning. AI, SL, EAT, and DMO contributed to manuscript writing. BM mentored the study team, guided study design and implementation, and contributed to the analysis and interpretation of the data.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
This research was made possible by the UK Medical Research Council and UK Department for International Development BREATHE-Africa MRC Partnership Grant, MR/L009242/1, the Government of Uganda’s National ICT Initiative Support Program (NIISP) of the Ministry of ICT and National Guidance and the MakCHS – UCBerkeley –Yale Pulmonary Complications of AIDS Research Training (PART) Program, NIH D43TW009607, from the Fogarty International Center.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Conflicts of interest
Charles Batte is part of the team that developed the Wulira app.
References
- The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5:291-360.
- [CrossRef] [Google Scholar]
- Consolidated Guidelines on Tuberculosis Treatment Geneva: World Health Organization; 2019.
- [Google Scholar]
- Guideline on Treatment of Drug Resistant Tuberculosis Geneva: World Health Organization; 2011.
- [Google Scholar]
- Reduced chance of hearing loss associated with therapeutic drug monitoring of aminoglycosides in the treatment of multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61:e01400-16.
- [CrossRef] [PubMed] [Google Scholar]
- An audiological profile of patients infected with multi-drug resistant tuberculosis at a district hospital in KwaZulu-Natal. S Afr J Commun Disord. 2016;63:e1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis. 2002;6:622-7.
- [Google Scholar]
- Hearing loss in patients on treatment for drug-resistant tuberculosis. Eur Respir J. 2012;40:1277-86.
- [CrossRef] [PubMed] [Google Scholar]
- AMTAS(®): Automated method for testing auditory sensitivity: II. Air conduction audiograms in children and adults. Int J Audiol. 2011;50:434-9.
- [CrossRef] [PubMed] [Google Scholar]
- Validated smartphone-based apps for ear and hearing assessments: A review. JMIR Rehabil Assist Technol. 2016;3:e13.
- [CrossRef] [PubMed] [Google Scholar]
- The new age of play audiometry: Prospective validation testing of an iPad-based play audiometer. J Otolaryngol Head Neck Surg. 2013;42:21.
- [CrossRef] [PubMed] [Google Scholar]
- Wulira App: Simple and Robust Hearing Assessment Application. Available from: https://www.wuliraapp.com [Last Accessed on 2020 Aug 04]
- [Google Scholar]
- Hearing threshold status and risk estimate of hearing impairment among administrative workforce. Indian J Occup Environ Med. 2018;22:11-6.
- [CrossRef] [PubMed] [Google Scholar]
- Potential Health Risks of Exposure to Noise from Personal Music Players and Mobile Phones Including a Music Playing Function. 2008. Available from: http://www.ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_018.pdf.In [Last Accessed on 2020 Sep 13]
- [Google Scholar]
- Sensorineural hearing loss in patients with multidrug-resistant tuberculosis: Case studies. Acta Otolaryngol Case Rep. 2017;2:96-102.
- [CrossRef] [Google Scholar]
- Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection. Int J Tuberc Lung Dis. 2018;22:667-74.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of hearing loss in multi-drug resistant tuberculosis (MDR-TB) patients undergoing aminoglycoside treatment. Int J Res Med Sci. 2015;3:1734-40.
- [CrossRef] [Google Scholar]
- Hearing loss in children treated for multidrug-resistant tuberculosis. J Infect. 2013;66:320-9.
- [CrossRef] [PubMed] [Google Scholar]
- Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear Nose Throat Disord. 2007;7:5.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing Loss at High Frequencies and Oxidative Stress: Anew Paradigm for Different Etiologies In: An Excursus into Hearing Loss Stavros Hatzopoulos and Andrea Ciorba. London: IntechOpen; 2018.
- [Google Scholar]
- Human cochlear pathology in aminoglycoside ototoxicity--a review. Acta Otolaryngol Suppl. 1987;436:117-25.
- [CrossRef] [PubMed] [Google Scholar]
- Aminoglycoside-induced hearing loss in HIV-positive and HIV-negative multidrug-resistant tuberculosis patients. S Afr Med J. 2012;102:363-6.
- [CrossRef] [PubMed] [Google Scholar]
- Smartphone hearing screening with integrated quality control and data management. Int J Audiol. 2014;53:841-9.
- [CrossRef] [PubMed] [Google Scholar]
- Affordable headphones for accessible screening audiometry: An evaluation of the Sennheiser HD202 II supra-aural headphone. Int J Audiol. 2016;55:616-22.
- [CrossRef] [PubMed] [Google Scholar]
- Biological calibration for web-based hearing tests: Evaluation of the methods. J Med Internet Res. 2014;16:e11.
- [CrossRef] [PubMed] [Google Scholar]






