Translate this page into:
Risk factors associated with the severity of pneumonia in a cohort of hospitalized children in a rural setting
*Corresponding author: Mohammed Mikhail Barday, Department of Paediatrics and Child Health, Stellenbosch University, Cape Town, Western Cape, South Africa barday@sun.ac.za
-
Received: ,
Accepted: ,
How to cite this article: Barday MM, Slogrove AL, Engelbrecht AL. Risk factors associated with the severity of pneumonia in a cohort of hospitalized children in a rural setting. J Pan Afr Thorac Soc 2022;3:130-9.
Abstract
Objectives:
Pneumonia remains a leading cause of death in South African children under 5 years of age. Known risk factors for pneumonia have been the focus of public health strategies to mitigate disease. This study aimed to determine whether adverse household environmental factors were associated with severe compared to non-severe pneumonia in children admitted to Worcester Provincial Hospital (WPH), South Africa.
Materials and Methods:
We conducted a case–control study at WPH from January 1st to December 31st, 2019, including children aged 0–59 months admitted with pneumonia. Using the WHO definition, children were categorized as having severe or non-severe pneumonia. Structured interviews with consenting primary caregivers were conducted in both groups on weekdays throughout the year to collect demographic, social, maternal, infant, and household factors. We compared the odds of adverse household environmental factors including tobacco smoke exposure, indoor smoke exposure, and overcrowding in children with severe compared to non-severe pneumonia.
Results:
A total of 305 children were included, 134 (43.9%) cases with severe pneumonia and 171 (56.1%) controls with non-severe pneumonia. Baseline characteristics of children, including age (median 6.9 months; IQR 2.5–17.5), appropriate nutritional status (81.6%; n = 249), and HIV unexposed and uninfected status (81.3%; n = 248), were similar between groups. Caregiver characteristics, including age (median 28 years; IQR 23–33), secondary schooling (71.2%, n = 217), and HIV negative status (81%, n = 247), were also comparable between groups. There was no association in univariable or multivariable analysis between severe pneumonia and adverse household environmental factors including tobacco smoke exposure (aOR 0.73; 95% CI 0.44–1.21), overcrowding (aOR 0.65, 95% CI 0.39–1.08), and indoor smoke exposure (aOR 2.85; 95% CI 0.89–9.09). However, children with severe pneumonia had at least 5 times greater odds (aOR 5.42; 95% CI 1.10–26.65) of living in a household with a pit latrine toilet compared to any other toilet than children with non-severe pneumonia.
Conclusion:
Few factors were found to be associated with pneumonia severity, except for living in a household with a pit latrine toilet. This may represent socioeconomic vulnerability and the risk associated with developing severe pneumonia.
Keywords
Childhood pneumonia
Severe pneumonia
Risk factors
Western Cape
South Africa
Paediatrics
Environmental health
INTRODUCTION
Pneumonia is a major contributor to childhood mortality and morbidity in developing countries.[1,2] The past decade has seen important declines in pneumonia-related mortality and to a lesser extent pneumonia-related incidence.[1] From 2005 to 2015, the total number of under 5 deaths globally due to pneumonia decreased by 36.9% (95% uncertainty interval [UI] 31.6–42.0) from an estimated 1.11 million (95% UI 1.03–1.20 million) to 0.703 million (95% UI 0.651–0.763 million).[1] However, the incidence of pneumonia only declined from 0.18 episodes per child year (95% UI 0.61–0.20) in 2005 to 0.15 episodes per child year (95% UI 0.13–0.17) in 2015.[1] In South Africa, pneumonia remains the leading single cause of death in children under 5 years of age accounting for 8.6% of child deaths.[3]
There are many well-recognized risk factors for pneumonia including adverse birth outcomes (being born preterm, low birth weight, or small for gestational age), young age, incomplete vaccination status, and malnutrition. Patients with severe pneumonia may have multiple risk factors acting in synergy resulting in more severe disease manifestation. The global and national trend toward the reduced burden of disease caused by pneumonia in children under 5 years highlights the important progress made in preventative strategies. Notable interventions include decreased indoor and ambient air pollution, improved childhood nutrition, greater coverage of childhood vaccines, progress made in HIV treatment, and case management of pneumonia.[1,2]
Evidence relating to household risk factors for pneumonia remains sparse in the South African context. Combustion of biomass fuels resulting in household air pollution (HAP), overcrowding, and maternal smoking is associated with an increased risk of pneumonia.[4-9] HAP is estimated to cause a high burden of morbidity and mortality globally, and of importance is the associated risk of lower respiratory tract infections (LRTIs).[4] A systematic review and meta-analysis demonstrated that passive smoking in the family home is associated with a risk of LRTI in infants.[10] South African studies including the Drakenstein Child Health Study and a case-control study conducted at Chris Hani Baragwanath Academic Hospital observed that tobacco smoke exposure was an independent risk factor for pneumonia.[7,8] Overcrowding is also a well-described risk factor for pneumonia.[5-7] These household risk factors, independently or interdependently, may play a significant role in the development of pneumonia. Whether they are associated with the severity of pneumonia in this population remains unknown. South African primary health care has focused on immunizations, nutrition, and case management of HIV and TB. However, there is limited understanding of household environmental factors and related interventions. Understanding the role of environmental risk factors associated with severe pneumonia may provide insight into areas that require immediate intervention in resource-limited settings.
The aim of this study was to determine factors associated with the severity of pneumonia in hospitalized children in a rural setting, with a primary focus on household environmental factors and secondarily child and caregiver factors.
MATERIALS AND METHODS
We conducted an unmatched case-control study of children aged 0–59 months admitted with severe pneumonia (cases) compared to non-severe pneumonia (controls). The study took place at WPH (Worcester, South Africa) between January 1st and December 31st, 2019. WPH is a regional public hospital, which provides specialist support to district hospitals and is located in the Breede Valley, approximately 110 km from Cape Town.
Eligible participants were children aged 0–59 months admitted with respiratory symptoms suggestive of clinical pneumonia. The pediatric ward and intensive care unit (ICU) admissions registers were reviewed during weekdays for eligible candidates. We conducted structured interviews with consenting primary caregivers, in a language of the caregivers’ choice, detailing information related to the child, primary caregiver, and household risk factors. Medical information related to pneumonia, anthropometric data, and HIV status were collected from the admission notes and supplemented by a review of the Road to Health Booklet. Laboratory investigations and/ or chest X-rays were performed at the discretion of the treating clinical team and no additional study-specific investigations were requested.
The primary outcome of pneumonia was classified according to the WHO clinical definition of pneumonia as severe or non-severe following a review of the completed admission notes.[11] Severe pneumonia was defined as cough or difficulty breathing and age-appropriate threshold for tachypnea or chest indrawing AND any one of the following features: hypoxemia (oxygen saturation of <92% in room air), respiratory support (i.e. high flow oxygen, CPAP, ventilatory support, and ICU admission), OR any integrated management of childhood illnesses general danger signs. Non-severe pneumonia was defined as cough or difficulty breathing AND age appropriate threshold for tachypnea or chest indrawing in the absence of hypoxia and any danger signs AND not requiring respiratory support. Although non-severe pneumonia in isolation did not warrant admission, children with comorbidities and those requiring supplemental oxygen but not hypoxic were admitted for observation and therapy. The diagnosis of bronchiolitis was based on clinical features manifesting as cough and wheeze.[12,13] The diagnosis of bronchopneumonia was based on clinical and radiological evidence of pneumonia characterized by suppurative inflammation localized in patches around bronchi which may or may not be localized to a single lobe of the lung.[14] Asthma was diagnosed clinically as recurrent and reversible episodes of bronchial obstruction manifesting in shortness of breath and wheeze that responded to bronchodilators.[12,13] The discharge diagnosis was based on cumulative information during the admission, including clinical presentation, inpatient records, and any additional investigations.
Clinical records and laboratory results were used to determine HIV status. The WHO classification of breastfeeding was used to categorize patients as not breastfed, partially breastfed, predominantly breastfed, or exclusively breastfed. Nutritional status was classified using sex-appropriate WHO child growth charts as appropriate weight-for-height, underweight-for-age, stunted, or wasted (moderate or severe).[15]
Indoor smoke exposure refers to biomass fuel used to generate energy for the purposes of cooking, heating, and lighting. Indoor tobacco smoke exposure refers to any household member that smoked including caregivers. Household crowding was defined as more than two people per household room.[16] This excluded bathrooms but included kitchen and living room areas.
Data were entered into a primary data collection tool developed in Research Electronic Data Capture (REDCap).[17] Basic descriptive statistics were conducted using IBM SPSS Statistics (Version 27). The association between severe pneumonia and adverse household environmental factors – tobacco smoke exposure, indoor smoke exposure, and overcrowding –was evaluated using the parametric Chi-square test if assumptions were met or the non-parametric Fisher’s Exact test when assumptions were not met. Unadjusted odds ratios (ORs) of the primary and secondary exposures were calculated comparing children with severe and non-severe pneumonia using univariable logistic regression. We additionally conducted multivariable logistic regression to estimate adjusted ORs including a priori identified confounders of age, sex, preterm birth, breastfeeding, nutrition status, HIV infection and exposure, participant comorbidities, immunization status, caregiver age and level of education, and additional factors associated with an outcome at P < 0.1. Using different multivariate regression models, we assessed the association of the adverse household environmental factors (indoor tobacco smoke exposure, overcrowding, and indoor biomass fuel exposure) individually and in combination.
Ethics approval was obtained from the Health Research Ethics Committee of Stellenbosch University and the Provincial Government of the Western Cape Health Impact Assessment.
RESULTS
Between January 1st and December 31st, 2019, we enrolled 305 children, of which 134 (43.9%) were categorized as severe pneumonia and 171 (56.1%) as non-severe pneumonia [Figure 1].
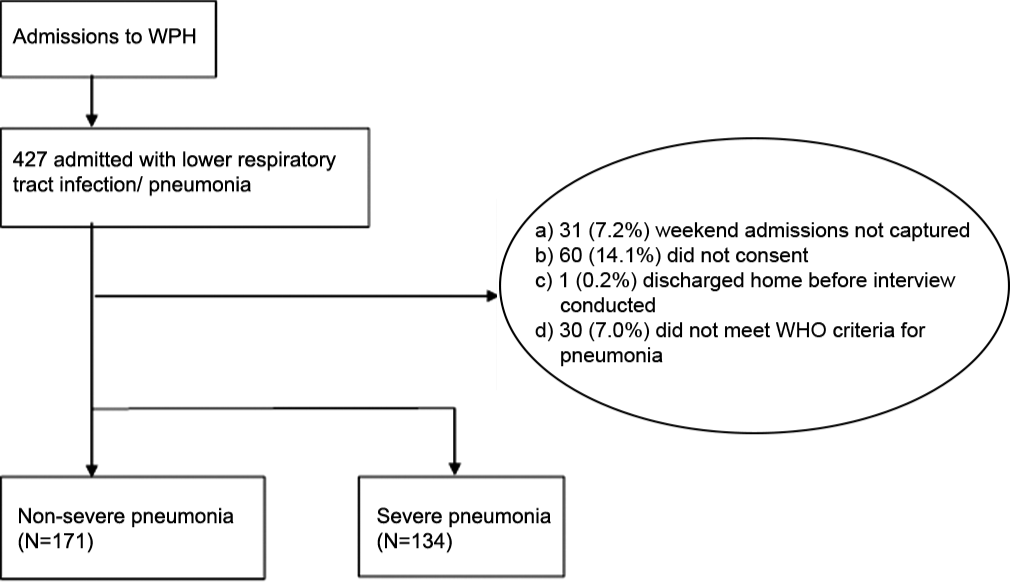
- Flow diagram of participant inclusion.
Child characteristics
Baseline characteristics of children [Table 1] were similar in both groups of participants with severe pneumonia and non-severe pneumonia. More than three-quarters of the participants (75.7%; n = 231) were younger than 18 months with 66.9% (n = 204) younger than 12 months of age. More than half the participants were born term (65.5%, n = 200) and over a quarter preterm (28.1%, n = 86). Breastfeeding practice was similar between groups with the majority of caregivers reporting exclusive breastfeeding (51.5%; n = 157). The majority of patients had appropriate nutritional status (81.6%, n = 249) with a small proportion being underweight for age (14.1%, n = 43) and stunted (18.4%, n = 56).
| Children – n(%) | Total (n=305) | Non-severe pneumonia (n=171) | Severe pneumonia (n=134) | P-valuea |
|---|---|---|---|---|
| Age (months) – median (IQR) | 6.9 (2.5–17.5) | 6.9 (2.8–16.0) | 6.9 (2.3–18.4) | 0.82b |
| Male sex – n(%) | 164 (53.8%) | 90 (52.6%) | 74 (55.2%) | 0.65 |
| Preterm n(%) | 86 (28.1%) | 52 (30.4%) | 34 (25.3%) | 0.31 |
| Late pretermc | 53 (17.4%) | 31 (18.1%) | 22 (16.4%) | 0.80 |
| Early pretermd | 33 (10.8%) | 21 (12.3%) | 12 (8.9%) | 0.12 |
| Small for gestational age | 52 (17.0%) | 31 (18.1%) | 21 (15.7%) | 0.53 |
| Nutritional status – n(%) | ||||
| Underweight | 43 (14.1%) | 24 (14.0%) | 19 (14.2%) | 0.97 |
| Stunted | 56 (18.4%) | 28 (16.4%) | 28 (20.9%) | 0.31 |
| Malnutrition status – n(%) | ||||
| Moderate acute malnutritione | 30 (9.8%) | 17 (9.9%) | 13 (9.7%) | 0.94 |
| Severe acute malnutritionf | 6 (2.0%) | 0 (0.0%) | 6 (4.5%) | <0.01 |
| Immunization up to date – n(%) | 265 (86.9%) | 152 (88.9%) | 113 (84.3%) | 0.50 |
| HIV status – n(%) | ||||
| Exposed uninfected | 47 (15.4%) | 27 (15.8%) | 20 (14.9%) | 0.55 |
| Exposed but not confirmed negative | 6 (1.9%) | 2 (1.2%) | 4 (3.0%) | |
| HIV infected | 4 (1.3%) | 1 (0.6%) | 3 (2.2%) | |
| Primary caregiver* – n(%) | ||||
| Age of primary caregiver – median (IQR) | 28.1 (23.2-33.9) | 27.8 (23.3-34.2) | 29.3 (23.0-33.7) | 0.98b |
| Highest level of education – n(%) | ||||
| Some secondary | 217 (71.1%) | 114 (66.7%) | 103 (76.9%) | 0.35 |
| Matriculated | 63 (20.7%) | 40 (23.4%) | 23 (17.2%) | |
| Employed – n(%) | 138 (45.2%) | 80 (46.8%) | 58 (43.3%) | 0.54 |
| Caregiver smoker – n(%) | 93 (30.5%) | 57 (33.3%) | 36 (26.9%) | 0.22 |
| Number of pack years (IQR) | 1.5 (0.6–2.5) | 1.8 (0.7–3.5) | 1.0 (0.48–1.9) | 0.02b |
| Caregiver with HIV – n(%) | 57 (18.7%) | 30 (17.5%) | 27 (20.1%) | 0.58 |
IQR: Interquartile range, CSG: Child Support Grant, CDG: Care Dependency Gran, HIV: Human immunodeficiency virus. *Unknown variable in one participant with severe pneumonia, aChi-squared test, bMann–Whitney U test, cEarly preterm birth: <34 weeks gestation, dLate preterm birth: <37 weeks but greater than or equal to 34 weeks gestation, eModerate acute malnutrition: weight for height Z-score <−2 standard deviations (SD) and >−3 SD, OR mid-upper arm circumference (MUAC) between 11.5 cm and 12.5 cm,[15]fSevere acute malnutrition: weight for height Z-score of <−3 SD, OR the presence of nutritional edema, OR mid-upper arm circumference (MUAC) of <11.5 cm[15]
Age-appropriate immunization coverage was recorded in 86.9% (n = 265) of children with no significant differences between groups. Children who were HIV exposed or HIV infected were similar between groups with 75.4% (43/57) on appropriate cotrimoxazole prophylaxis.
The final diagnosis in 78.3% (n=239) of children was pneumonia or bronchopneumonia [Table 2]. The most prevalent co-morbidity was congenital heart disease occurring in 4.6% (n=14) of all children with pneumonia. The most frequent concomitant diagnoses included anaemia (3.9%; n=12), failure to thrive[18] (3.3%; n=10), acute gastroenteritis (3.0%; n=9) and urinary tract infection (2.6%; n=8).
| Diagnosis | Total (n=305) | Non-severe pneumonia (n=171) | Severe Pneumonia (n=134) | P-value |
|---|---|---|---|---|
| Diagnostic classification – n(%)* | ||||
| Pneumonia | 107 (35.1%) | 54 (31.6%) | 53 (39.6%) | 0.14 |
| Bronchopneumonia | 132 (43.3%) | 77 (45.0%) | 55 (41.0%) | 0.48 |
| Asthma | 11 (3.6%) | 5 (2.9%) | 6 (4.5%) | 0.69 |
| Bronchiolitis | 32 (10.5%) | 22 (12.9%) | 10 (7.5%) | 0.24 |
| Pulmonary tuberculosis | 53 (17.4%) | 33 (19.3%) | 20 (14.9%) | 0.45 |
| Other | 46 (15.1%) | 18 (10.5%) | 28 (20.9%) | - |
| Concomitant diagnoses and Comorbidities – n (%)* | ||||
| None | 258 (84.6%) | 149 (87.1%) | 109 (81.3%) | 0.16 |
| Congenital heart disease | 14 (4.6%) | 6 (3.5%) | 8 (6.0%) | 0.45 |
| Anemia | 12 (3.9%) | 6 (3.5%) | 6 (4.5%) | 0.66 |
| Failure to thriveg | 10 (3.3%) | 4 (2.3%) | 6 (4.5%) | 0.29 |
| Urinary tract infection | 8 (2.6%) | 1 (0.6%) | 7 (5.2%) | 0.01 |
| Acute gastroenteritis | 9 (3.0%) | 6 (3.5%) | 3 (2.2%) | 0.51 |
Caregiver characteristics
Maternal characteristics were comparable among both groups of children with severe pneumonia and non-severe pneumonia [Table 1]. The majority of caregivers had received some secondary schooling (71.1%, n = 217); however, only 20.7% (n = 63) matriculated with no differences between groups. Eighteen percent (n = 57) of caregivers were known to have HIV with no statistical difference between groups. Overall, 30% (n = 93) of caregivers reported current smoking, with slightly fewer smokers in the severe pneumonia group compared to the non-severe pneumonia group, 26.9% (n = 36) and 33.3% (n = 57), respectively (P = 0.22). The number of pack years was also less in the severe pneumonia group compared to the non-severe pneumonia group, with median pack years of 1.0 (IQR 0.48–1.9) and 1.8 (IQR 0.70–3.50), respectively (P = 0.02).
Household risk factors
The majority of participants (58.7%, n = 179) lived in formal dwellings; 93.1% (n = 284) had access to electricity and 62.6% (n = 191) reported water piped into the dwelling [Table 3]. Exposure to household tobacco, in household members other than the primary caregiver, was assessed in 269 participants, of whom 55% (n = 148) reported household members that smoked. Open fires were reported in 40.6% (124/305) of households with wood (97.6%, 121/124) followed by coal (12.1%, 15/124) being the most frequently used biomass fuel. There was no association between severe pneumonia and non-severe pneumonia in households that made open fires versus those that did not.
| Total (n=305) | Non-severe pneumonia (n=171) | Severe pneumonia (n=134) | P-valuea | |
|---|---|---|---|---|
| Formal dwelling – n(%) | 179 (58.7%) | 106 (62.0%) | 73 (54.5%) | 0.18 |
| Electricity available – n(%) | 284 (93.1%) | 160 (93.6%) | 124 (72.5%) | 0.58 |
| Drinking water – n(%) | ||||
| Public tap | 55 (18.0%) | 30 (17.5%) | 25 (14.6%) | 0.80 |
| Piped into yard | 56 (18.4%) | 30 (17.5%) | 26 (15.2%) | 0.67 |
| Piped into dwelling | 191 (62.6%) | 109 (63.7%) | 82 (61.2%) | 0.64 |
| Toilet type – n(%) | ||||
| Flush toilet | 269 (88.2%) | 153 (89.5%) | 116 (86.6%) | 0.43 |
| Pit toilet | 11 (3.6%) | 2 (1.2%) | 9 (6.7%) | 0.01 |
| No facility | 14 (4.6%) | 8 (4.7%) | 6 (4.5%) | 0.93 |
| Mobile toilet | 10 (3.3%) | 8 (4.7%) | 2 (1.5%) | 0.12 |
| Communal toilet – n(%) | 79 (25.9%) | 41 (24.0%) | 38 (28.4%) | 0.38 |
| Open household fire material*** | 124 (40.7%) | 74 (43.3%) | 50 (37.3%) | 0.23 |
| Wood (subgroup) | 121 (97.6%) | 71 (95.9%) | 50 (100.0%) | 0.45 |
| Coal (subgroup) | 15 (12.1%) | 5 (6.8%) | 10 (20.0%) | 0.06 |
| Stove type – n(%) | ||||
| Electric | 263 (86.2%) | 148 (86.5%) | 115 (85.8%) | 0.85 |
| Gas | 45 (14.8%) | 28 (16.4%) | 17 (12.7%) | 0.36 |
| Household members – median (IQR) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 4.0 (4.0–6.0) | 0.04b |
| Members/room – median (IQR) | 1.5 (1.3–2.3) | 1.7 (1.3–2.5) | 1.5 (1.3–2.0) | 0.17b |
| Overcrowding – n(%) | 120 (39.3%) | 73 (42.7%) | 47 (35.1%) | 0.17 |
| Household smokers** – n(%) | 148 (55.0%) | 90 (58.8%) | 58 (50.0%) | 0.28 |
| Open household fires – n(%) | 124 (40.6%) | 74 (43.3%) | 50 (37.3%) | 0.29 |
IQR: Interquartile range. **Household smoking unknown in 18 participants in the non-severe pneumonia group and 17 participants in the severe pneumonia group, ***Patients could select more than one biomass fuel burned in their household. Therefore, the subcategory sums up to more than 100% of the total number that reported burning items in the household. aChi-squared test, bMann-Whitney U test
A median of 5 (IQR 4.0–6.0) household members were reported living together with a median of three members (IQR 3.0–4.0) sleeping in the same room as the participant. Overcrowding was reported in 120 households (39.3%) with no significant difference between groups.
In the unadjusted analyses, there was no association between indoor tobacco smoke exposure, overcrowding or indoor biomass fuel exposure, and severe pneumonia [Table 4]. Adjusting for factors known to be associated with severe pneumonia in children, there was still no association between our hypothesized adverse household environmental factors and the odds of severe pneumonia. However, children with severe pneumonia had at least a 5 times greater odds (aOR 5.42; 95% CI 1.10–26.65) of living in a household with a pit latrine toilet compared to any other toilet than children with non-severe pneumonia.
| uOR (95% CI) | Model 1: Indoor Smoking aOR (95% CI) | Model 2: Overcrowding aOR (95% CI) | Model 3: Indoor Smoke Exposure aOR (95% CI) |
Model 4: Combined aOR (95% CI) |
|
|---|---|---|---|---|---|
| Indoor tobacco smoking – any (reference none) |
0.73 (0.46–1.16) |
0.68 (0.42–1.11) |
----------------- | ----------------- | 0.73 (0.44–1.21) |
| Overcrowding -yes (reference no) |
0.72 (0.45–1.15) |
----------------- | 0.63 (0.38–1.03) |
----------------- | 0.65 (0.39–1.08) |
| Indoor coal biomass fuel burned (reference no biomass burned) |
2.31 (0.75–7.02) |
----------------- | ----------------- | 2.58 (0.83–8.00) |
2.85 (0.89–9.09) |
| Indoor non-coal biomass fuel burned (reference no non-coal biomass burned) |
1.49 (0.91–2.43) |
----------------- | ---------------- | 0.64 (0.392–1.07) |
0.71 (0.423–1.19) |
| Child age <3 months (reference >3 months) |
1.41 (0.86–2.32) |
1.39 (0.82–2.36) |
1.32 (0.78–2.23) |
1.35 (0.79–2.29) |
1.31 (0.77–2.25) |
| Birth weight <1500g (reference >1500g) |
1.14 (0.42–3.04) |
1.10 (0.40–3.01) |
1.19 (0.43–3.27) |
1.25 (0.46–3.41) |
1.27 (0.46–3.51) |
| Any breastfeeding (reference no breastfeeding) |
1.00 (0.48–2.11) |
1.02 (0.48–2.18) |
1.00 (0.47–2.13) |
1.08 (0.50–2.33) |
1.08 (0.50–2.34) |
| Immunizations not up to date (reference up to date) |
1.49 (0.75–2.94) |
1.46 (0.72–2.96) |
1.42 (0.70–2.88) |
1.49 (0.73–3.03) |
1.43 (0.70–2.93) |
| Child with HIV (reference without HIV) |
3.89 (0.40–37.85) |
3.43 (0.34–34.05) |
4.23 (0.42–42.54) |
3.67 (0.36–36.92) |
4.18 (0.41–42.73) |
| Maternal age <20 years (reference >20 years) |
1.04 (0.52–2.07) |
1.08 (0.53–2.23) |
0.98 (0.48–2.01) |
1.01 (0.49–2.07) |
1.06 (0.51–2.23) |
| Caregiver education less than matric (reference at least matric) |
0.70 (0.40–1.21) |
0.75 (0.42–1.32) |
0.72 (0.40–1.28) |
0.73 (0.41–1.30) |
0.64 (0.38–1.16) |
| Household toilet pit latrine (reference any other toilet) |
6.08 (1.29–28.65) |
5.01 (1.02–24.62) |
5.18 (1.05–25.41) |
5.21 (1.06–25.57) |
5.42 (1.10–26.65) |
Different multivariate regression models were used to assess the association of the adverse household environmental factors (indoor tobacco smoke exposure, overcrowding and indoor biomass fuel exposure) individually and in combination. aOR: Adjusted odds ratio, CI: Confidence interval, uOR: Unadjusted odds ratio, HIV: Human Immunodeficiency Virus
DISCUSSION
Pneumonia remains an important public health burden and determining factors that may mitigate severe disease have beneficial implications in resource limited settings. These factors may help inform interventions and policy decisions in an already constrained health-care system.
This case–control study compared risk factors of cases with severe pneumonia to unmatched controls with non-severe pneumonia admitted to a regional secondary level rural hospital. We compared infant, caregiver, and household risk factors and found a predominantly homogenous group of participants with few significant variations between cases with severe pneumonia and controls with non-severe pneumonia. These similarities were noted in baseline characteristics including a similar education level with most caregivers having achieved some secondary education or matriculated and residing in formal dwellings. Established risk factors for pneumonia were also similar between groups, with the majority of children being less than 7 months of age at the time of admission, almost a quarter born preterm, 87% appropriately immunized and just over half having been exclusively breastfed for 6 months. The distinguishing feature among these groups related to children with severe pneumonia having greater odds of living in a household with a pit latrine toilet compared to children with non-severe pneumonia.
As expected, a large proportion of participants were infants in the 1st year of their life. Severe pneumonia and non-severe pneumonia in the early months of life reflect the vulnerability of this young age group which is consistent with studies in low-to-middle income countries.[8,19,20] Our study, however, did not demonstrate group differences related to age, indicating that young age, though a known risk factor for pneumonia, was not a factor associated with the severity of pneumonia in this study. More than a quarter of participants were preterm with the majority of participants appropriate for gestational age. Prematurity is associated with immature immune function, reduced production of pro-inflammatory cytokines, and reduced passive immunity conferred by transplacental maternal antibodies.[21-23] Despite prematurity being a known risk factor for pneumonia,[23,24] it was not associated with greater odds of severe disease when comparing severe pneumonia with non-severe pneumonia. This was demonstrated in both late and early preterm births. These findings differ from that of the Drankenstein Child Study which demonstrated that age <2 months and preterm birth were significantly associated with serious outcomes.[24] This discrepancy may be due to the difference in study design and the population sampling used.
According to the WHO definition of pneumonia, all children with evidence of severe acute malnutrition and features of respiratory tract infection are considered to have severe pneumonia; thus, we could not evaluate severe acute malnutrition as an independent risk factor for severe pneumonia. Despite evidence to support the importance of malnutrition as a risk factor for pneumonia,[25,26] there was no observable association with the severity of disease. This observation may be artifactual due to assigning severely malnourished children automatically to the severe pneumonia group and essentially excluding them from analysis.
Prevention of childhood communicable diseases through immunization plays an important role in reducing pneumonia mortality, morbidity, and burden of disease.[27,28] This important preventative strategy, however, was not associated with severe pneumonia in this study with similar and fairly good immunization coverage reported in the severe pneumonia and non-severe pneumonia groups. In addition, routine immunizations do not cover the most common causes of pneumonia such as respiratory syncytial virus, parainfluenza virus, and adenovirus.[20] Although determining the infective agent was not part of this study, the young study cohort would have been more vulnerable to these viruses.
While HIV-infection was low (1.3%), HIV exposure without infection was fairly prevalent (17.3%) in this study. Our study did not demonstrate an association with HIV infection or HIV exposure without infection and severe pneumonia. This subgroup may have been too small to demonstrate smaller differences between groups, requiring a greater sample. Alternatively, this may reflect the strength of the PMTCT program in this community and possible immune reconstitution in patients living with HIV reducing the risk of severe disease.
Tobacco smoke exposure is a well described risk factor for pneumonia in studies conducted in South Africa and Cameroon.[7,8,29] The Drakenstein Child Health Study, a birth cohort study conducted outside of Cape Town, demonstrated in regression models that maternal smoking was an independent risk factor for pneumonia. The Drakenstein Study included ambulatory and hospitalized LRTIs and was conducted at two separate clinics. This study used WHO criteria to define pneumonia similar to our study, but the clinic setting and ambulatory LRTI were a noted difference.[30] A case–control study conducted at Chris Hani Baragwanath Academic Hospital also observed that a primary caregiver who smoked was a risk factor for pneumonia (aOR: 5.15, 95% CI: 2.94– 9.03).[7] This study, however, did not use the WHO criteria for pneumonia and was conducted in a tertiary hospital. The study conducted in Cameroon assessed pneumonia risk factors using a cross-sectional study at a secondary hospital. This study included both ambulatory and hospitalized acute respiratory tract infections and found that passive smoking was associated with acute respiratory tract infections. There were notable differences in study design and sample selection in all three studies. These differences in study design and sample selection may represent different populations with different risk factors and disease severity compared to the cohort admitted to WPH and included in our study.
Our study reflected less smoking in caregivers in the severe pneumonia group than the non-severe pneumonia group. This unexpected observation may have been due to reverse causality bias implicated in the study design and behavior change following hospital admission of participants. Caregivers may have felt guilty about volunteering accurate information related to smoking habits and underestimated the exact amount of cigarettes smoked. This may have been more pronounced in the severe pneumonia group causing behavioral change and reporting socially desirable information rather than that increased tobacco smoke exposure may be protective.
Our study did not demonstrate that household smoke exposure was associated with the severity of pneumonia. Although the burning of coal for household cooking, heating, or light demonstrated increased odds of developing severe pneumonia, it was not statistically significant however, the study may have been under-powered to identify this difference.
Overcrowding, although an established risk factor for pneumonia, was not associated with the severity of pneumonia. In contrast, our study demonstrated that having a greater number of household members was associated with reduced odds of developing severe disease. This may be due to larger households being able to provide more childhood monitoring, stimulation, caregiving, education, and financial support in this generally socioeconomically vulnerable population.
In relation to the primary study objective, no association was demonstrated in univariable or multivariable analysis between adverse household environmental factors and severe pneumonia. Severe pneumonia was only associated with greater odds in households that reported use of pit latrine toilets. This association may represent the most indigent of this community and their increased risk of developing severe disease. Further analysis of socioeconomic disparities may be helpful in assigning risk to other adverse household environmental factors and the detection of protective factors. Investigations into the accessibility to clean water, sanitation, and waste disposal or the lack thereof would provide important information about the socioeconomic circumstances that this community experiences and the risk of disease. It is also interesting to note that a greater association of severe disease was demonstrated in those that reported use of pit latrine toilet as opposed to those having no toilet facility. This may be related to methods of waste disposal, hygiene, and sanitation around pit latrine facilities.
Caregivers that reported no toilet facilities may also have had access to alternative amenities such as their neighbor’s lavatory or rented facilities. Further inquiry into these circumstances are needed to shed more light on the matter.
In an attempt to help inform interventions and policy decisions to mitigate pneumonia severity, we continue to advocate for primary health interventions. Primary interventions such as childhood immunizations, maternal education relating to smoking and smoke exposure, exclusive breastfeeding, appropriate education on nutrition, and compliance with vertical HIV transmission prevention guidelines remain important strategies to reduce community respiratory disease burden. Parallel strategies to improve living conditions and alleviate poverty through appropriate housing, access to sustainable energy, clean water, sanitation, and appropriate sewerage disposal are of equal importance and complement interventions.
Limitations
Notable limitations that deserve consideration include the method of data capturing, the broad pneumonia case definition, assessing the severity of pneumonia, and quantifying household environmental factors.
The method of data collection was dependent on information recalled by the primary caregiver and some elements may have been inaccurate. This may have influenced certain questions related to past events such as the duration of breastfeeding or previous hospital admissions as well as reporting on household exposures, which would ideally have been directly observed by the investigators.
An important limitation related to study sampling and feasibility was the exclusion of outpatient pneumonia cases. Although not part of the study design, this group of patients represents an important source of information related to pneumonia and risk factors for developing pneumonia as opposed to risk factors driving the severity of disease.
A larger study cohort would have been better powered to detect smaller but still clinically meaningful differences between population groups. This was not feasible within the time and study resources available.
The WHO definition of severe pneumonia may not have been a sensitive tool to distinguish severe and non-severe pneumonia in an in-hospital setting. Patients requiring admission may already have fallen into a spectrum of severe disease that was not differentiated when using the WHO definition of non-severe pneumonia.
The diagnosis of pneumonia was based on clinical findings in children that were documented by the attending clinician. This element may have varied amongst the attending doctors when assessing the severity of pneumonia. The use of dedicated physicians recording the clinical features at presentation and during admission may have improved the accuracy of classifying pneumonia.
The WHO definition of pneumonia casts a broad diagnostic net. Respiratory tract infections that imitate pneumonia include asthma and bronchiolitis. These share the same clinical manifestations as the pneumonia case definition. Although these are not pneumonia events (i.e., lung parenchymal disease) they were not excluded from the study which may have marginally affected our results related to risk factors. While other study definitions for pneumonia exist, using the WHO definition allows us to compare findings with studies that have utilized the same classification and may be more applicable in resource limited settings.[30]
HAP and tobacco smoke exposure were not quantified in terms of particulate matter concentrations, carbon monoxide levels, and the duration of indoor smoke exposure. Confounding factors including indoor ventilation, population density, and environmental air pollution which may be a biproduct of biomass fuels were not measured. Although these factors did not fall into the scope of this study, this information may have been of value when comparing severe pneumonia and non-severe pneumonia.
CONCLUSION
This cohort provides a comparison of risk factors in children admitted with severe pneumonia and non-severe pneumonia in a rural setting in South Africa. We compared child, caregiver, and household risk factors and found a predominantly homogenous group of participants with few significant variations. These similarities were noted in both baseline characteristics and risk factors. The distinguishing feature among these groups related to children with severe pneumonia having greater odds of living in a household with a pit latrine toilet compared to children with non-severe pneumonia. This may represent the socioeconomic differences of this cohort and the risk associated with developing severe pneumonia in the most marginalized and vulnerable part of the population.
Acknowledgment
The authors would like to thank Professor Sharon Kling for her guidance and support that helped shape the manuscript. To Mrs. Sithole who was a welcomed research assistant who assisted with interviews and translation for Xhosa-speaking participants. To Prof Tonya Esterhuizen, from the Division of Epidemiology and Biostatistics, Stellenbosch University, who advised and guided statistical analysis. To Worcester Provincial Hospital (WPH) and the Provincial Government of the Western Cape Department of Health who endorsed this study and allowed us to conduct research.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Research assistance was provided through the INFORM HIV Free project funded by the US National Institutes of Health (R21HD093531).
Conflicts of interest
There are no conflicts of interest.
References
- Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1133-61.
- [CrossRef] [Google Scholar]
- Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388:3027-35.
- [CrossRef] [Google Scholar]
- Statistical Release Mortality and Causes of Death in South Africa, 2008: Findings from Death Notification. Statistics South Africa 2016
- [Google Scholar]
- Millions dead: How do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185-206.
- [CrossRef] [PubMed] [Google Scholar]
- Household air quality risk factors associated with childhood pneumonia in urban Dhaka, Bangladesh. Am J Trop Med Hyg. 2014;90:968-75.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:10401.
- [CrossRef] [Google Scholar]
- Risk Factors for Presumed Bacterial Pneumonia Among HIV-uninfected Children Hospitalized in Soweto, South Africa. Pediatr Infect Dis J. 2016;35:1169-74.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: The Drakenstein Child Health Study. Lancet Glob Health. 2015;3:e95-103.
- [CrossRef] [Google Scholar]
- The association between environmental tobacco smoke exposure and childhood respiratory disease: A review. Expert Rev Respir Med. 2017;11:661-73.
- [CrossRef] [PubMed] [Google Scholar]
- Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: Systematic review and meta-analysis. Respir Res. 2011;12:5.
- [CrossRef] [PubMed] [Google Scholar]
- Department of Maternal NC and AH, World Health Organization In: Revised Who Classification and Treatment of Pneumonia in Children at Health Facilities: Evidence Summaries. Geneva: World Health Organization; 2014.
- [Google Scholar]
- Training Course on Child Growth Assessment. 2008. Geneva: World Health Organisation; 7 Available from: https://www.apps.who.int/iris/bitstream/handle/10665/43601/9789241595070_C_eng.pdf?sequence=3&isAllowed=y [Last accessed on 2022 Jan 19]
- [Google Scholar]
- Children's Access to Housing. 2015. Available from: http://www.ci.uct.ac.za/sites/default/files/image_tool/images/367/Child_Gauge/2006/Child_Gauge_2016-children_count_housing.pdf [Last accessed on 2022 May 30]
- [Google Scholar]
- Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical case definitions for classification of intrathoracic tuberculosis in children: An update. Clin Infect Dis. 2015;61(Suppl 3):S179-87.
- [CrossRef] [PubMed] [Google Scholar]
- The predictive performance of a pneumonia severity score in human immunodeficiency virus-negative children presenting to hospital in 7 low-and middle-income countries. Clin Infect Dis. 2020;70:1050-7.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet. 2019;394:757-79.
- [Google Scholar]
- Neonatal monocytes exhibit a unique histone modification landscape. Clin Epigenetics. 2016;8:99.
- [CrossRef] [PubMed] [Google Scholar]
- Host components contributing to respiratory syncytial virus pathogenesis. Front Immunol. 2019;10:2152.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with serious outcomes of pneumonia among children in a birth cohort in South Africa. PLoS One. 2021;16:e0255790.
- [CrossRef] [PubMed] [Google Scholar]
- Pneumonia in severely malnourished children in developing countries mortality risk, aetiology and validity of WHO clinical signs: A systematic review. Trop Med Int Health. 2009;14:1173-89.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal and child nutrition: Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427-51.
- [CrossRef] [Google Scholar]
- Effectiveness of pneumococcal conjugate vaccine and rotavirus vaccine introduction into the South African public immunisation programme. S Afr Med J. 2014;104:228-34.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulm Med. 2018;18:7.
- [CrossRef] [PubMed] [Google Scholar]
- Lower respiratory tract infections in children in a well-vaccinated South African birth cohort: Spectrum of disease and risk factors. Clin Infect Dis. 2019;69:1588-96.
- [CrossRef] [PubMed] [Google Scholar]







