Translate this page into:
Prevalence and predictors of pulmonary fungal infections in patients with acute leukemia and aggressive lymphomas: Implications for cancer care in developing countries
*Corresponding author: Adaeze Chikaodinaka Ayuk, Department of Pediatrics, College of Medicine University of Nigeria Enugu Campus and University of Nigeria Teaching Hospital, Ituku, Enugu, Nigeria. adaeze.ayuk@unn.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Ayuk AC, Ekop E, Ozoya O, Lawal O, Emole J. Prevalence and predictors of pulmonary fungal infections in patients with acute leukemia and aggressive lymphomas: Implications for cancer care in developing countries. J Pan Afr Thorac Soc 2021;2:154-60.
Abstract
Objectives:
Among patients receiving cancer therapy, pulmonary fungal infections (PFIs) are an important cause of morbidity and mortality. Identifying predictors of PFI can direct targeted prophylaxis to improve outcomes, especially in low- and middle-income countries (LMIC) with limited resources. The objectives of the study were to evaluate the predictors of PFI in hospitalized patients with hematological malignancies in the United States and implications for prioritizing anti-fungal care in LMIC.
Materials and Methods:
Using the 2018 National Inpatient Sample, we conducted a retrospective study of patients ≥18 years, with acute leukemia or aggressive lymphoma. Demographics and outcomes were compared between patients with and without PFI. Predictors of PFI were evaluated by regression analysis.
Results:
PFI was diagnosed in 1635 (0.8%) of 205,525 eligible hospitalizations and aspergillosis was noted in 1315 (80.4%) of PFI cases. Patients with acute myeloid leukemia (AML) accounted for 64.2% of cases of PFI. Patients with PFI, when compared with those without PFI, were younger, had higher Charlson comorbidity index, were more likely to be non-Caucasian, and to have AML. Patients with PFI had higher odds of respiratory failure, sepsis, and in-hospital mortality. Variables associated with PFI were Hispanic or native American origin (OR = 1.71; 95% CI: 1.21–2.42), Charlson comorbidity index ≥3 (OR = 1.52; 95% CI: 1.16–2.00), neutropenia (OR = 1.97; 95% CI: 1.58–2.46), malnutrition (OR = 2.30; 95% CI: 1.75–3.01), bone marrow transplant status (OR = 2.28, 95% CI: 1.53–3.39), and AML diagnosis (OR = 3.12; 95% CI: 2.40–4.05).
Conclusions:
This study identified variables associated with PFI in patients diagnosed with acute leukemia and aggressive lymphomas. In LMIC, where resources are scarce, patients with cancer who have the identified high-risk characteristics should be given priority for antifungal prophylaxis.
Keywords
Pulmonary
Fungal infections
Aspergillosis
Leukemia
Lymphoma
INTRODUCTION
Fungi rarely cause lower respiratory tract infections in immunocompetent patients because the host anatomical and innate immune defenses clear the fungal elements and prevent clinical disease.[1,2] In patients with hematological malignancies, the pulmonary mucociliary defense may be impaired by chemotherapy or radiation, predisposing patients to fungal infections. Moreover, such patients may experience neutropenia from defective marrow function or cancer therapy, thereby further heightening their risks of invasive fungal infections. Pulmonary fungal infections (PFIs) can present primarily in the lungs or as manifestation of disseminated fungal infections.
PFIs account for most of the invasive fungal diseases reported in the literature. A report from the Center of Expertise in Mycology in the Netherlands shows that a majority of adults and children evaluated for severe fungal infections suffered from pulmonary infections or signs/symptoms.[3] In a multicenter trial of posaconazole and voriconazole for the treatment of adult patients at risk for invasive aspergillosis, 80% of study patients had infections limited to the lower respiratory tract (primarily lung).[4] Estimates from Nigeria among study population 12 years to 60 years showed 40% prevalence rate of pneumocystis pneumonia in children and 5-year period prevalence of 60,377 cases of chronic pulmonary aspergillosis in patients with pulmonary tuberculosis.[5]
Due to the poor outcomes reported with invasive fungal infections,[6-8] antifungal prophylaxis is increasingly being recommended for patients considered to be high risk for these infections. The Infectious Diseases Society of America has provided guidelines for antifungal prophylaxis in the following high-risk groups: patients undergoing intensive chemotherapy for acute myeloid leukemia or myelodysplastic syndromes, allogeneic stem cell transplant recipients, patients with prior history of invasive aspergillosis, and patients with anticipated prolonged neutropenia.[9] The National Comprehensive Cancer Network recommends antifungal prophylaxis for patients with increased risk of fungal infections during cancer therapy.[10] Unfortunately, universal adoption of these antifungal prophylaxis recommendations is almost impossible because practices are usually guided by local epidemiology, availability of drugs, and other economic conditions seen in low- and middle-income countries (LMICs).[11,12]
A good understanding of the risk factors associated with PFI is therefore necessary if health professionals must improve outcomes and efficiently allocate resources. We thus aimed to evaluate the current prevalence and predictors of PFI in patients with cancer in a high-income country to further extrapolate what predictors may serve as guide for prioritizing antifungal prophylaxis where resources may be scarce.
MATERIALS AND METHODS
Data from the 2018 National Inpatient Sample (NIS) were used for the retrospective analyses. The NIS is the largest publicly available all-payer inpatient care database in the United States (US), sponsored by the Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. The NIS is designed to produce regional and national estimates of inpatient utilization, access, charges, quality, and outcomes in the USA. As a stratified sample, the 2018 NIS contains 20% (over 7 million) of all discharges from non-federal acute care hospitals in the US for the year 2018. When weighted, it estimates more than 35 million hospitalizations nationally. The 2018 NIS sampling frame includes data from 48 state-wide data organizations (47 states plus the district of Columbia).[13] Each discharge record in the NIS contains patient-level (demographics, diagnoses, procedures, and mortality) as well as hospital-level data. Further information is available at https://www.hcup-us.ahrq.gov.
Ethical clearance
The Institutional Review Board of Henry Ford Hospital, Detroit, Michigan, determined that a formal review of this study was not required, and all researchers underwent a US certification to use data for this retrospective review.
Study design
This was a 1-year retrospective study of all hospitalized patients aged 18 years and older, who had a diagnosis of acute leukemia or aggressive lymphomas (acute myeloid leukemia [AML], acute lymphoblastic leukemia, diffuse large B-cell lymphoma, Burkitt lymphoma, primary mediastinal large B-cell lymphoma, mantle cell lymphoma, and Hodgkin’s disease). Eligible hospitalizations were selected using the International Statistical Classification of Disease, Tenth Revision (ICD-10) codes[14] for acute leukemia and aggressive lymphomas. Documented diagnoses of PFI were identified using the respective ICD-10 codes. The PFIs of interest were pulmonary candidiasis, pulmonary aspergillosis, pulmonary cryptococcus, and pulmonary mucormycosis. Patient-level and hospital-level variables were extracted from eligible hospital records. Patient-level characteristics obtained were age, gender, race, insurance type, income, and comorbidities while the hospital-level characteristics were hospital location, bed size, and teaching status. Charlson comorbidity index was used as a summary measure of patients’ comorbidities because it has been confirmed to be a useful substitute for individual comorbidity variables in health services research.[15] Malnutrition was defined as any diagnosis of cachexia, or mild, moderate, or severe protein-calorie malnutrition. Acute respiratory failure was defined as any requirement for endotracheal intubation or mechanical ventilation. Income was represented by the median household income quartiles for patients’ zip code. For 2018, the median income quartiles were defined as: $1–$45,999, $46,000–$58,999, $59,000–$78,999, and ≥$79,000 (US dollars).[13] Demographics, comorbidities, and inhospital mortality were compared between cancer patients with and without PFI. The primary study outcome was prevalence of PFI. Secondary study outcomes were health-care utilization and mortality associated with PFI, as well as clinical predictors associated with PFI.
Statistics
Baseline patient demographics, comorbidities, and hospital characteristics were summarized using counts and proportions for categorical variables and mean, standard deviation (SD), and range for continuous variables. As the NIS database is a stratified sample, discharge level weights provided by the HCUP, were applied to all analyses to obtain national estimates. In compliance with the data use agreement, categories were combined, and some data were suppressed to avoid reporting any cell counts <11. Chi-squared (χ2) test was used to compare categorical variables while continuous variables were compared using one-way analysis of variance. Univariable and multivariable regression analyses were performed to identify predictors that were associated with PFI, and odds ratio (OR) and confidence intervals (CIs) were reported. All tests were two sided, and results were considered significant at the 95% level (P < 0.05). Statistical analysis was performed with Stata version 16 (StataCorp, College Station, TX, USA).
RESULTS
During the study period, a total of 205,525 hospitalizations met the inclusion criteria for the specified hematological malignancies. This cohort included 118,300 (57.5%) males, 142,223 (69.2%) Caucasians, and 18,641 (9.1%) African-Americans. The mean (SD) age of the study participants was 59.2 (±17.8) years, with a range of 18–90 years. The most common malignancies seen were non-Hodgkin’s lymphoma in 91,253 (44.4%) and AML in 69,262 (33.7%) patients. Up to 1248 (0.6%) of patients in the cohort had more than 1 hematological malignancy. Chemotherapy was administered in 56,930 (27.7%) hospitalizations, and 19,936 (9.7%) of the study participants had received bone marrow transplantation (BMT). A total of 177,985 (86.6%) hospitalizations were in urban teaching hospitals.
The diagnosis of PFI was made in 1635 hospitalizations, resulting in a prevalence of 0.8%. Of the patients with PFI, aspergillosis was noted in 1315 (80.4%) of cases while candidiasis was noted in 195 (11.9%) cases. The distribution of PFI based on causative pathogens is shown in [Figure 1].
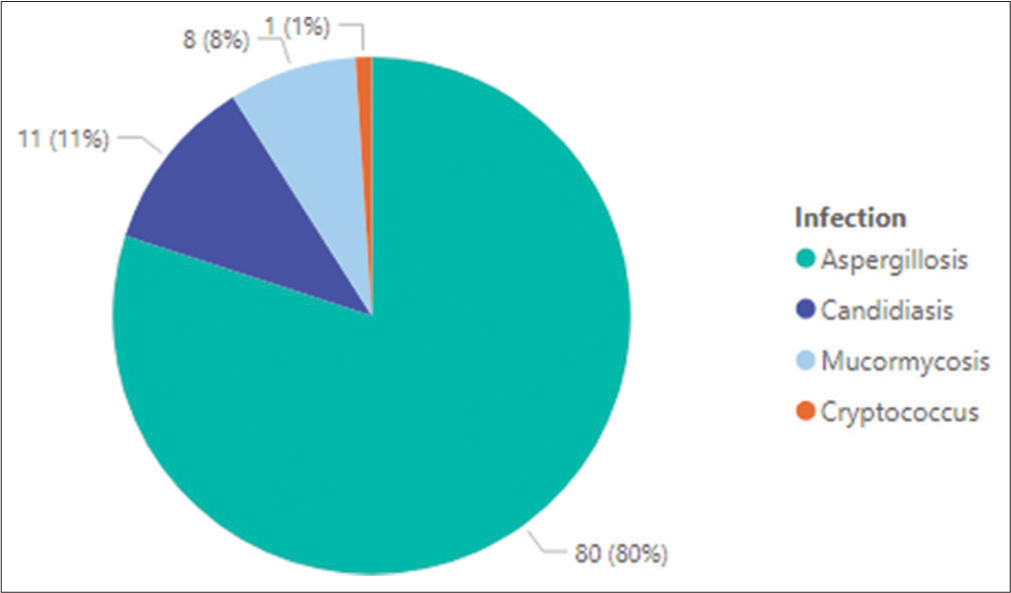
- Distribution of pulmonary fungal infections in study cohort.
AML was the primary hematological malignancy in 1050 (64.2 %) of those who had PFI. Clinical variables compared between patients with and without PFI are presented in [Table 1].
| PFI (n=1635) |
No PFI (n=203,890) |
P | |
|---|---|---|---|
| Age, mean, years | 56.8 | 59.3 | 0.013 |
| Sex, n(%) | |||
| Female | 625 (38.2) | 86592 (42.5) | 0.185 |
| Race, n(%) | 0.023 | ||
| Caucasian | 1013 (61.9) | 141,194 (69.2) | |
| African-American | 137 (8.4) | 18,493 (9.1) | |
| Hispanic | 278 (17.0) | 25,262 (12.4) | |
| Asian/Pacific Islander | 106 (6.5) | 7911 (3.9) | |
| Others | 101 (6.2) | 11,010 (5.4) | |
| Missing | 0 | 20 | |
| Charlson comorbidity index, n(%) | 0.002 | ||
| 0–2 | 595 (36.4) | 92,240 (45.2) | |
| ≥3 | 1040 (63.6) | 11,1650 (54.8) | |
| Hospital bed size, n(%) | 0.707 | ||
| Small | 165 (10.1) | 25,711 (12.6) | |
| Medium | 375 (22.9) | 42,164 (20.7) | |
| Large | 1095 (67.0) | 136,015 (66.7) | |
| Hospital teaching status, n(%) | 0.051 | ||
| Rural or urban non-teaching | 145 (8.9) | 27,444 (13.5) | |
| Urban teaching | 1490 (91.1) | 176,446 (86.5) | |
| Underlying hematological disorder, n(%) | |||
| Acute myeloid leukemia | 1050 (64.2) | 68,242 (33.5) | 0.000 |
| Acute lymphoblastic leukemia | 265 (16.2) | 27,464 (13.5) | 0.200 |
| Aggressive non-Hodgkin’s lymphoma | 285 (17.4) | 90,976 (44.6) | 0.000 |
| Hodgkin disease | 60 (3.7) | 18,289 (9.0) | 0.001 |
| >1 hematological disorder codes | 25 (1.5) | 1223 (0.6) |
PFI: Pulmonary fungal infection
The PFI group, compared to the non-PFI group, was younger (mean age 56.8 vs. 59.3 years, P = 0.013), more likely to be non-Caucasian (38.1% vs. 30.8%, P = 0.023), more likely to have Charlson comorbidity index ≥3 (63.6% vs. 54.8%, P = 0.002), more likely to have a diagnosis of AML (64.2% vs. 33.5%, P = 0.000), had longer hospital stay (mean length of stay of 23 vs. 9 days, P = 0.000), and had higher mean total hospital charges ($422,972 vs. $124,976, P = 000). As shown in [Table 2], in-hospital mortality rate was higher for the PFI group versus the non-PFI group (16.5% and 5.5%, respectively, P = 0.000). Similarly, patients with PFI had higher rates of bacteremia, sepsis, and organ failure (respiratory and renal) compared to patients without PFI.
| PFI n(%) |
No PFI n(%) |
P | |
|---|---|---|---|
| Respiratory failure | 225 (13.7) | 7034 (3.5) | 0.000 |
| Bacteremia | 110 (6.7) | 5668 (2.8) | 0.000 |
| Sepsis | 510 (31.2) | 30,685 (15.1) | 0.000 |
| Septic shock | 235 (14.4) | 9216 (4.5) | 0.000 |
| Acute renal failure | 495 (30.3) | 38,719 (19.0) | 0.000 |
| Anemia | 350 (21.4) | 47,649 (23.4) | 0.483 |
| Thrombocytopenia | 355 (21.7) | 35,640 (17.5) | 0.065 |
| Neutropenia | 545 (33.3) | 35,762 (17.5) | 0.000 |
| Discharge disposition | |||
| In-hospital mortality | 270 (16.5) | 11,296 (5.5) | 0.000 |
| Discharge to other facilities* | 210 (12.8) | 26,995 (13.2) | 0.848 |
PFI: Pulmonary fungal infection. *Short term, intermediate care, or skilled nursing facilities.
Univariable analysis revealed that PFI was associated with higher odds of acute respiratory failure requiring advanced respiratory support, bacteremia, sepsis, septic shock, and inhospital mortality [Table 3].
| OR | 95% CI | P | |
|---|---|---|---|
| Endotracheal intubation or mechanical ventilation | 4.46 | 3.02–6.60 | 0.000 |
| Bacteremia | 2.53 | 1.63–3.92 | 0.000 |
| Sepsis | 2.56 | 2.06–3.18 | 0.000 |
| Septic shock | 3.54 | 2.70–4.70 | 0.000 |
| Acute renal failure | 1.85 | 1.47–2.33 | 0.000 |
| Anemia | 0.89 | 0.65–1.22 | 0.483 |
| Thrombocytopenia | 1.31 | 0.98–1.74 | 0.066 |
| In-hospital mortality | 3.37 | 2.55–4.47 | 0.000 |
OR: Odds ratio, CI: Confidence interval
On multivariable analysis [Table 4], predictors associated with PFI were Hispanic or native American origin (OR = 1.71; 95% CI: 1.21–2.42), Charlson comorbidity index above ≥3 (OR = 1.52; 95% CI: 1.16–2.00), neutropenia (OR = 1.97; 95% CI: 1.58–2.46), malnutrition (OR = 2.30; 95% CI: 1.75–3.01), bone marrow transplant status (OR = 2.28; 95% CI:1.53–3.39), and a diagnosis of AML (OR = 3.42; 95% CI: 2.60–4.50). In a multivariable model adjusting for confounders, PFI was an independent predictor for inhospital mortality (OR = 2.33; 95%CI: 1.74–3.13, P = 0.000).
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Female gender | 0.81 | 0.62–1.06 | 0.118 |
| Age<65 years | 1.31 | 0.93–1.85 | 0.120 |
| Charlson comorbidity index≥3 | 1.52 | 1.16–2.00 | 0.003 |
| Hispanic or native American origin | 1.71 | 1.21–2.42 | 0.002 |
| COPD or asthma | 1.17 | 0.86–1.60 | 0.307 |
| Disorders of iron metabolism | 1.73 | 0.52–5.76 | 0.371 |
| Neutropenia | 1.97 | 1.58–2.46 | 0.000 |
| Malnutrition | 2.30 | 1.75–3.01 | 0.000 |
| Bone marrow transplant status | 2.28 | 1.53–3.39 | 0.000 |
| Diagnosis of AML | 3.12 | 2.40–4.05 | 0.000 |
COPD: Chronic obstructive pulmonary disease, AML: Acute myeloid leukemia, OR: Odds ratio, CI: Confidence interval
DISCUSSION
Among patients receiving cancer therapy, PFIs are an important cause of morbidity and mortality as shown in this current study. Even though PFIs constitute a significant proportion of invasive fungal infections, few studies have comprehensively described the burden of PFI in patients with hematologic malignancies. In this present study, we leveraged data from a large US inpatient database to evaluate prevalence, resource utilization, and outcomes of PFI. Our data show low prevalence, but significant morbidity and mortality from PFI in patients with acute leukemia and aggressive lymphomas. Our study also identified several risk factors for PFI and confirmed PFI as a driver for higher health-care resource use.
PFI prevalence rates reported in the literature vary with the population being studied, the climate, and the clinical practice patterns. In our study cohort, a PFI prevalence of 0.8% is plausible given that antifungal prophylaxis for high-risk patients is a common practice in the US.[9,10] In other clinical contexts, where prophylaxis is not routine or conditions are more favorable for fungal growth, prevalence rates up to 20% are typical.[16-20] When breakthrough infections are further factored into the prevalence, the burden is higher among high-risk patients. A study in Nigeria evaluated 216 participants with presumptive tuberculosis, where 62% were male while 10% were children <20 years and they reported a PFI prevalence of 73.6%, with aspergillosis being the predominant pulmonary fungal pathogen.[21] Their reported risk factors for PFI were: presence of PTB, prolonged (>3 weeks) use of antibiotics, pet possession, and cigarette smoking. Although not assessed in the current study, studies done in LMIC should assess for these important additional risk factors in patients at risk of PFI.
In studies where patients admitted to the intensive care unit were the target study population, the rates of invasive aspergillosis ranged from 0.33% to as high as 19.0%.[22-29] Higher rates of aspergillosis reported in lung malignancies can be explained by likely tumor infiltration, mucosal injury inflicted by radiotherapy, and immunosuppression from chemotherapy.[30-33]
The causative fungal organisms and underlying malignancies found in our study are consistent with what has been reported.[34-37] Aspergillosis was the most common type of PFI in our cohort as has been reported by others.[16,34-37] In a few reports where Candida species were the prevalent cause of PFI, the population studied were either not predominantly cancer patients[38] or had a low proportion of PFI.[6] Other fungal infections reported in our study were Candidiasis, Mucormycosis, and Cryptococcus, and these are typical pathogens among immunosuppressed patients.[34,39] In comparison to other hematological malignancies, AML poses a higher risk for invasive fungal infections.[16,40-42]
Clinical variables found to increase the odds of PFI in our study were BMT status, neutropenia, and acute myeloid leukemia. These are likely due to the impairment of both cell line of defense against fungal infections which are the macrophages and neutrophils,[30] with the degree of immune suppression associated with odds of PFI from our study. Furthermore, chemotherapy, as well as immunosuppressive agents given for BMT, can both induce neutropenia and cause pulmonary mucociliary impairment, predisposing these patients to fungal infections. The patients may also experience neutropenia from defective marrow function, thereby further heightening their risks of invasive fungal infections. Hispanic or native American origin, malnutrition, and multiple comorbidities were also associated with higher odds. Characteristics such as malnutrition, poorly managed multiple comorbidities, and origin from a minority population group have been found by many studies to negatively affect health and mortality.[43-47] Neutropenia and malnutrition are known risk factors for fungal infections,[12,43].Unfortunately, these characteristics reflect the health conditions of many cancer patients living in LMIC.
Interestingly, a prior history of asthma or chronic obstructive pulmonary disease was not a significant risk factor for PFI, an observation that has been reported by at least one other study.[30]
As expected, patients with PFI were severely ill and had increased risks for bacteremia, sepsis, septic shock, renal, and respiratory failure. Consequently, these patients had longer hospital stay, hospital costs, and mortality. Although the burden of cancer is increasing globally, a significant portion of this burden is borne by LMICs.[48-51]
While access to cancer care remains one of the greatest needs in LMIC, other drivers of worse cancer outcomes include poor health-seeking behavior of patients, high out-of-pocket medical costs, insufficient training and retention of oncology professionals, unavailability of novel cancer therapeutics, diagnostics, and infrastructure.[52] Because cancer research is not a top priority for many governments of LMIC, very few patients receive cancer treatments within the context of well-designed clinical trials. For the oncology health professionals in LMIC, lack of access to up-to-date medical literature and cancer care guidelines may preclude the use of appropriate medications even when they are available. As such, cancer care in many parts of LMIC is largely driven by availability and affordability rather than standard guidelines.
Regardless of the peculiar circumstances in any LMIC, patients with hematological malignancies can be successfully treated, with careful planning. Cancer therapies should be administered within or in proximity to facilities that have the trained staff or are equipped to care for critically ill patients since such patients are prone to developing severe complications which may require intensive care as was noted in the current study. If available, antifungal prophylaxis should especially be offered to patients at high risk for fungal infections. Empiric antifungal therapy should include a mold-active agent pending microbiological confirmation of causative agent. Therapeutic drug monitoring and molecular typing of strains may become necessary if compliance, breakthrough infection or drug toxicity is of concern. All nutritional deficiencies and comorbidities should be properly addressed in patients undergoing cancer treatments. If cancer patients develop PFI, they will likely require multidisciplinary care from oncologists, infectious disease physicians, intensivists, and pulmonologists, among others. Provision of medications and cancer research ought to be high priority for policy-makers in LMIC.
There are some limitations to our study. Due to the retrospective design, we could not determine if PFIs were limited to the lungs or a part of disseminated fungal disease. Second, administrative databases like the NIS lack data on medication use, and so the use of antifungal prophylaxis by all study participants could not be firmly ascertained, and thus, it is uncertain that these cases were primary fungal infection or breakthrough infections. Finally, our study design could not distinguish between probable, possible, and proven fungal infections.
CONCLUSION
In the current era of increasing use of prophylactic antifungals in at-risk patients, our study shows that PFIs remain a major cause of morbidity and mortality in patients with acute leukemia and aggressive lymphomas. Where resources are scarce, patients with the identified high-risk characteristics should be given priority for antifungal prophylaxis, and surveillance with clinical, molecular, and drug monitoring measures when possible. In LMIC, patients with these hematological malignancies should ideally be managed at referral centers equipped for critically ill patients, due to associated medical complications.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pathophysiological aspects of Aspergillus colonization in disease. Med Mycol. 2019;57(Suppl 2):S219-27.
- [Google Scholar]
- Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. 2017;15:661-74.
- [CrossRef] [Google Scholar]
- A multidisciplinary approach to fungal infections: One-year experiences of a center of expertise in mycology. J Fungi (Basel). 2020;6:274.
- [CrossRef] [Google Scholar]
- Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: A phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021;397:499-509.
- [CrossRef] [Google Scholar]
- Epidemiology and clinical features of invasive fungal infection in a us health care network. Open Forum Infect Dis. 2018;5:ofy187.
- [Google Scholar]
- Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Clin Microbiol Infect. 2015;21:490.e1-10.
- [Google Scholar]
- Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176-84.
- [CrossRef] [Google Scholar]
- Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the infectious diseases society of America. Clin Infect Dis. 2011;52:e56-93.
- [Google Scholar]
- Prevention and Treatment of Cancer-Related Infections (Version2.2020) 2021. https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf [Last accessed on 2021 Apr 22]
- [Google Scholar]
- Economic considerations of antifungal prophylaxis in patients undergoing surgical procedures. Ther Clin Risk Manag. 2011;7:13-20.
- [Google Scholar]
- Clinical practice update of antifungal prophylaxis in immunocompromised children. Rev Esp Quimioter. 2019;32:410-25.
- [Google Scholar]
- ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision (2nd ed). Geneva: World Health Organization; 2004.
- [Google Scholar]
- Why summary comorbidity measures such as the charlson comorbidity index and elixhauser score work. Med Care. 2015;53:e65-72.
- [Google Scholar]
- The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica. 2006;91:1068-75.
- [Google Scholar]
- Epidemiology, management, and outcome of invasive fungal disease in patients undergoing hematopoietic stem cell transplantation in China: A multicenter prospective observational study. Biol Blood Marrow Transplant. 2015;21:1117-26.
- [CrossRef] [Google Scholar]
- Clinical risk score for invasive fungal diseases in patients with hematological malignancies undergoing chemotherapy: China assessment of antifungal therapy in hematological diseases (CAESAR) study. Front Med. 2019;13:365-77.
- [CrossRef] [Google Scholar]
- High incidence of invasive fungal infection during acute myeloid leukemia treatment in a resource-limited country: Clinical risk factors and treatment outcomes. Support Care Cancer. 2019;27:3613-22.
- [CrossRef] [Google Scholar]
- Incidence of invasive fungal infections in acute myeloid leukemia without antifungal prophylaxis. Clin Lymphoma Myeloma Leuk. 2020;20:e883-9.
- [Google Scholar]
- Spectrum of pulmonary fungal pathogens, associated risk factors, and anti-fungal susceptibility pattern among persons with presumptive tuberculosis at Gombe, Nigeria. Int J Mycobacteriol. 2020;9:144-9.
- [Google Scholar]
- Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33:23-32.
- [CrossRef] [Google Scholar]
- Comparison of premortem clinical diagnoses in critically iII patients and subsequent autopsy findings. Mayo Clin Proc. 2000;75:562-7.
- [CrossRef] [Google Scholar]
- Post mortem examination in the intensive care unit: Still useful? Intensive Care Med. 2004;30:2080-5.
- [Google Scholar]
- Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: A 6-year survey. Clin Infect Dis. 2006;43:577-84.
- [CrossRef] [Google Scholar]
- Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621-5.
- [CrossRef] [Google Scholar]
- Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: Risk factors, clinical presentation and outcome. Crit Care. 2005;9:R191-9.
- [Google Scholar]
- Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care. 2006;10:R31.
- [Google Scholar]
- A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Intensive Care Med. 2003;29:1981-8.
- [CrossRef] [Google Scholar]
- Invasive pulmonary aspergillosis: Comparative analysis in cancer patients with underlying haematologic malignancies versus solid tumours. J Hosp Infect. 2020;104:358-64.
- [CrossRef] [Google Scholar]
- Invasive aspergillosis in the intensive care unit. Clin Infect Dis. 2007;45:205-16.
- [CrossRef] [Google Scholar]
- Unusual forms of subacute invasive pulmonary aspergillosis in patients with solid tumors. J Infect. 2014;69:387-95.
- [CrossRef] [Google Scholar]
- Invasive aspergillosis in patients with solid tumors. Cancer. 2004;101:2300-2.
- [CrossRef] [Google Scholar]
- Pulmonary fungal infection with yeasts and pneumocystis in patients with hematological malignancy. Ann Med. 2005;37:259-69.
- [CrossRef] [Google Scholar]
- Epidemiology of invasive pulmonary aspergillosis In: Aspergillosis: From Diagnosis to Prevention (1st ed). Berlin: Springer; 2009.
- [Google Scholar]
- Invasive fungal infections in patients with hematologic malignancies (aurora project): Lights and shadows during 18-months surveillance. Int J Mol Sci. 2012;13:774-87.
- [CrossRef] [Google Scholar]
- Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: A retrospective cohort study. PLoS One. 2018;13:e0197851.
- [Google Scholar]
- Fungal isolates of the respiratory tract in symptomatic patients hospitalized in pulmonary units: A mycological and molecular epidemiologic study. J Multidiscip Healthc. 2020;13:661-9.
- [CrossRef] [Google Scholar]
- Fungal infection in lung transplantation. Transpl Infect Dis. 2002;4(Suppl 3):24-31.
- [Google Scholar]
- Significant alterations in the epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2008;88:508-15.
- [CrossRef] [Google Scholar]
- Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: An autopsy study over a 15-year period (1989-2003) Haematologica. 2006;91:986-9.
- [Google Scholar]
- An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: Diagnosis and therapeutic outcome. EORTC invasive fungal infections cooperative group. J Infect. 1998;37:173-80.
- [CrossRef] [Google Scholar]
- Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin Nutr. 2019;38:1310-6.
- [CrossRef] [Google Scholar]
- Mini nutritional assessment and 10-year mortality in free-living elderly women: A prospective cohort study with 10-year follow-up. Eur J Clin Nutr. 2012;66:1050-3.
- [CrossRef] [Google Scholar]
- Ethnicity and mortality in the United States: Individual and community correlates. Soc Forces. 1997;76:169-98.
- [CrossRef] [Google Scholar]
- Race/ethnicity and U.S. adult mortality: Progress, prospects, and new analyses. Du Bois Rev. 2011;8:5-24.
- [CrossRef] [Google Scholar]
- An empirical study of chronic diseases in the united states: A visual analytics approach. Int J Environ Res Public Health. 2018;15:431.
- [CrossRef] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [Google Scholar]
- Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.
- [Google Scholar]
- Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749-68.
- [Google Scholar]
- Haematological malignancies in Nigeria: Challenges in diagnosis and management-a systematic review. J Biomed Res Clin Pract. 2020;3:282-92.
- [Google Scholar]






