Translate this page into:
Point of care lung ultrasonographic findings in patients with clinical diagnosis of severe childhood community acquired pneumonia in the tropics
*Corresponding author: Dr. Janet A Akinmoladun, Department of Radiology, University College Hospital, Ibadan, Oyo state, Nigeria. jaakinmoladun@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Akinmoladun J, Atalabi OM, Falade AG, Mortimer K, Ogunniyi A. Point of care lung ultrasonographic findings in patients with clinical diagnosis of severe childhood community acquired pneumonia in the tropics. J Pan Afr Thorac Soc. 2024;5:17-25. doi: 10.25259/JPATS_16_2023
Abstract
Objectives:
This study aimed at defining the Lung ultrasonographic (LUS) features of severe Childhood community-acquired pneumonia (CAP) in under-5s with clinical pneumonia, as well as the correlation between clinical and ultrasonographic findings.
Materials and Methods:
A prospective descriptive study conducted at the Children Emergency Ward of the University College Hospital (UCH), Ibadan, Nigeria over a 1-year period. Children aged 1month to 59 months, with clinical features of severe pneumonia were recruited for the study. LUS was done at the bedside. Ethical approval was obtained for the study.
Results:
There were 86 children with mean age ± SD of 13.59 ± 15.55 and 50 (58.1%) males. The highest number of patients (56, 65.1%) were in the 1-12-month age group. Ultrasonographic signs of pneumonia were detected in 68 (79.1%) of the children; 55(64%) had consolidation, 29(23.3%) showed florid B-lines and 20 (23.3%) had pleural fluid. Duration of cough, and crackles on auscultation were the only features that showed significant association with the LUS diagnosis of pneumonia.
Conclusion:
LUS is a good point of care diagnosis of CAP in under-5 children and it can be safely done at the bedside. It is therefore recommended as a first line imaging modality in children with clinical suspicion of CAP in the Tropics.
Keywords
Pneumonia
Lung ultrasound scan
Children
INTRODUCTION
Childhood community-acquired pneumonia (CAP) is one of the leading causes of under-5 mortality globally, and it is the most common infectious cause of death among children worldwide.[1-3] A child dies from pneumonia every 43 s, and over 800,000 children under five die globally every year, or around 2,000 every day.[2]
It is estimated that CAP accounts for 18% of the total number of deaths in children below five years, which is more than pulmonary tuberculosis, acquired immune deficiency syndrome and malaria combined.[4] In Nigeria, CAP is the single largest cause of under-5 deaths, and it accounted for 140,520 (19%) of under-5 deaths in 2017.[5]
The World Health Organization (WHO) defines pneumonia based on simple clinical signs to enhance its early diagnosis, prompt treatment, and referral of severe cases from the first-level health facility.
The clinical signs used include cough, tachypnea, and lower chest in-drawing.[6] CAP is defined as pneumonia in a previously healthy child who acquired the infection outside a health facility or develops the illness within 48 hours of admission into a health facility. CAP is said to be severe when a child presents with at least one danger sign other than cough and/or difficulty breathing, which will warrant admission into the emergency unit. These signs include central cyanosis, severe respiratory distress, grunting with every breath, vomiting everything, loss of consciousness, or convulsions not due to meningitis or cerebral malaria.[6,7] Of all the childhood pneumonia cases in low- and middle-income countries (LMICs), only 8.7% are severe enough to require hospital admission.[8]
Pneumonia is considered a clinical diagnosis; however, this poses some challenges because the signs and symptoms vary depending on a child’s age.[6,7]
Chest computed tomography is the gold standard imaging for the diagnosis of pneumonia; however, it is not ethically accepted in children due to its high ionizing radiation. It is also very expensive, not affordable in resource-poor settings, and children need to be sedated.[9]
Chest radiography has been used traditionally for many years to complement clinical skills in the proper diagnosis and management of severe CAP. However, there is a global effort to reduce ionizing radiation in medical imaging of children to lessen the contribution of iatrogenic irradiation to the burgeoning cancer epidemic.[9-11]
In 1986, Weinberg et al.[12] introduced lung ultrasonography (LUS) as a new method of evaluating CAP. Many studies in the developed country have investigated its use in the evaluation of childhood pneumonia, and it has shown promising results. Some characteristic features that were identified are that it can be easily performed at the bedside, it is cheap, radiation-free, portable, and repeatable, and it can be performed by trained clinicians with immediate results.[11,13-15] These properties make it an attractive tool for resource-limited settings.[16]
Even though many studies have been done in well-resourced settings in high-income countries, the use of lung ultrasonography for the management of childhood pneumonia is yet to be widely accepted in sub-Saharan Africa, where most of the affected children are found.[4,7] The purpose of this study was to define the ultrasonographic appearances of severe pneumonia in children with clinical suspicion in our hospital and to evaluate the correlation between clinical and ultrasonographic findings. We hypothesized that lung ultrasonography can be a useful imaging modality in the management of childhood pneumonia in Nigeria.
MATERIALS AND METHODS
This was a prospective descriptive study conducted at the children’s emergency ward of the University College Hospital (UCH), Ibadan, Nigeria. The hospital is located in the southwestern part of Nigeria, and it is one of the major tertiary hospitals in the country.
The study spanned a period of 1 year, from December 2020 to November 2021.
Study population
Consecutive children, aged between 2 months and 60 months, admitted in the children’s emergency ward on account of severe pneumonia during the study period were recruited for the study.
Sample size calculation
The minimum sample size required was calculated using the formula for estimating single proportions.
N = Minimum sample size
Z = Standard normal deviate corresponding to a 5% level of significance (95% confidence interval) = 1.96
p = Expected proportion of CAP based on previous study (Orimadegun and Myer, 2019)
d is the level of precision = 5% = 0.05
N = Z2 ´ p(q-p) d2
Minimum sample size = 56.
Inclusion criteria
The following criteria were included in the study:
Children aged between 2 months and five years old who present with symptoms of pneumonia such as cough, difficulty with breathing (tachypnea, lower chest indrawing)
Children suspected of having pneumonia by the attending pediatrician
Parents or caregiver’s consent.
Exclusion criteria
The following criteria were excluded from the study:
Children with previous invasive chest interventions, for example, chest thoracostomy
Children otherwise unable to co-operate for LUS
Chest abnormalities and chest trauma with large dressings and subcutaneous emphysema
No parental/caregiver’s consent.
Clinical evaluation
A pro forma was used to collect the data and this was divided into two sections. The first section was for the sociodemographics of the child, and the information documented was the age and sex of the child as well as the weight in kilogram. The information in the second section of the pro forma included the symptoms the patient presented with and the physical signs on examination. The presence of the cough and its duration were documented. The vital signs recorded were the respiratory rate, the pulse rate, and the temperature. Tachypnea was defined as respiratory rates of 50 or more breaths per minute in children aged 2–11 months, as well as respiratory rates of 40 or more breaths per minute in children aged 12–59 months, according to the WHO cutoff criteria for age-adjusted tachypnea.[17] Other signs documented were the presence of lower chest wall indrawing, subcostal retraction, intercostal retraction, nasal flaring, and grunting. The presence of central cyanosis was determined by checking for blue tongue. Hypoxia was confirmed with an oximeter reading less than oxygen saturation (SpO2) of 90%. The pediatric resident doctor filled the first two sections at the children’s emergency unit.
Lung ultrasonography
This section was on lung ultrasound findings, and this was filled by the performing radiologist. The lung ultrasound scans were done within 48 hours of admission into the emergency ward.
A portable ultrasound unit Sonoscape E2 (manufactured in 2019 by Sonoscape Medical Corp, China) with a 5–10 MHz linear transducer was used to scan each subject, and a 3–6 MHz microconvex transducer was used in addition to the linear probe when indicated. The lung examinations were performed at the bedside by an expert pediatric radiologist. The patients were placed in either a supine or sitting position and each hemithorax was divided into three parts – anterior, lateral, and posterior and they were evaluated in both longitudinal and transverse sections.[18] Anterior and lateral parts of the hemithorax were examined with the patient in the supine position or sitting facing the radiologist, while the posterior part was examined with the patient lying prone or sitting with the back to the radiologist.
With the transthoracic approach, the pleura is visualized as a smooth, echogenic line called the pleural line [Figure 1]. The pleural line slides from side to side with respiration, and its absence is the main US criterion of pneumothorax. Beneath the pleural lines are parallel echogenic lines equally distanced from one another called A-lines [Figure 1]. These are reverberation artifacts. B-lines are linear echogenic reflections, arising from and are roughly vertical to the pleural line. The presence of 2 or 3 B-lines is a normal finding [Figure 1].
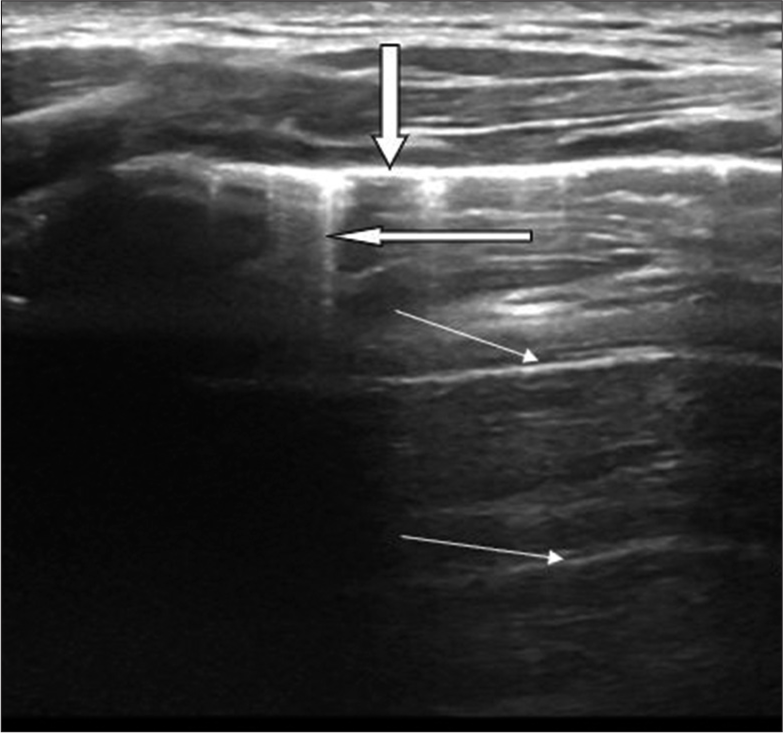
- Longitudinal scan of a normal lung showing a thick transverse echogenic line (downward pointing arrow) which represents the pleural line. Two short straight reflections of the pleural line consistent with A-lines are also seen (thin arrows). B-lines are seen as linear echogenic reflections arising from and are roughly vertical to the pleural line (thick transverse arrow).
Pneumonic consolidation was diagnosed when the normal A- and B-lines were replaced with a hypoechoic structure that looks like the liver (Hepatization). Air trapped within the bronchioles is seen as echogenic lines consistent with air bronchogram [Figure 2]. The presence of more than three separate B-lines (florid B-lines) seen at the same time in any view or when they become confluent, giving a white lung, has been correlated with thickening of the interlobular septae due to increased interstitial fluid [Figure 2].
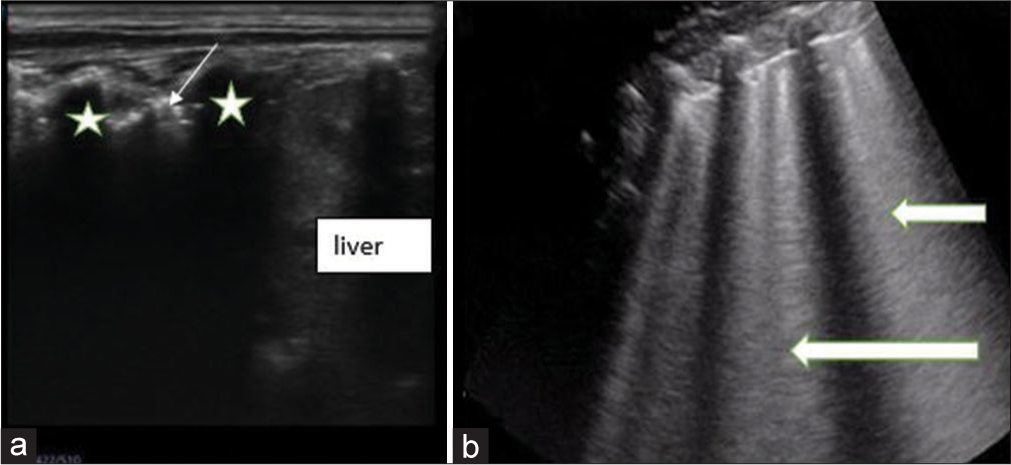
- Sagittal views on lung ultrasound of a child (a) shows the area of consolidation with air bronchogram seen as echogenic foci (slim arrow) within it. Acoustic shadows from the ribs are noted (*). While the liver is seen inferior to the ribs (b) shows florid B-lines (block arrows) arising from the pleural lines.
The presence of pleural collection was documented, and the collection was characterized as a transudate if completely sonolucent and exudate if there were internal echoes, hyperreflective foci of gas, or echogenic septa within it.
The diagnosis of pneumonia was made on ultrasound in this study if there was one or more of any of the following findings: consolidation, with or without air bronchogram, florid B-lines, or pleural effusion.[19]
Data analysis
All data were entered into SPSS version 20 (IBM, Armonk, New York, United States). Continuous variables were analyzed using descriptive statistics. The Chi-square test was used to test the association between quantitative variables. P < 0.05 was taken as the level of significance. Summary statistics are presented as appropriate.
RESULTS
A total of 86 children with mean age ± standard deviation (SD) of 13.59 ± 15.55 and 50 (58.1%) males clinically diagnosed with severe pneumonia were enrolled for the study [Table 1].
| Gender/age group | Frequency | Percentage |
|---|---|---|
| Gender | ||
| Male | 50 | 58.0 |
| Females | 36 | 42.0 |
| Age group (months) | ||
| 2–12 | 56 | 65.1 |
| 13–24 | 19 | 22.1 |
| 25–36 | 2 | 2.3 |
| 37–48 | 5 | 5.8 |
| 49–59 | 4 | 4.7 |
About one-fifth of the children (17, 19.8%) had a reoccurrence of the illness, of which the majority (10, 58.8%) had two or more re-occurrences. The majority of the children (67, 77.9%) had a history of cough with an average duration of 5 days. Similarly, most of them (73, 84.9%) had a history of fever [Table 2].
| Variables | Frequency | Median (IQR) | Percentage | Range |
|---|---|---|---|---|
| Symptoms | ||||
| First time of illness | ||||
| No | 17 | 19.8 | ||
| Yes | 69 | 80.2 | ||
| Number of re-occurrences | ||||
| None | 69 | 80.2 | 2–4 | |
| One | 7 | 8.1 | ||
| Two or more | 10 | 11.6 | ||
| History of cough | ||||
| Yes | 67 | 77.9 | ||
| No | 19 | 22.1 | ||
| Duration of cough in days | 5 (2.50; 8.50) | 1–12 | ||
| History of fever | ||||
| Yes | 73 | 84.9 | ||
| No | 13 | 15.1 | ||
| Signs | ||||
| Intercostal retraction | ||||
| Yes | 68 | 79.1 | ||
| No | 18 | 20.9 | ||
| Subcostal retraction | ||||
| Yes | 61 | 70.9 | ||
| No | 25 | 29.1 | ||
| Lower chest wall retraction | ||||
| Yes | 29 | 33.7 | ||
| No | 57 | 66.3 | ||
| Grunting | ||||
| Yes | 10 | 11.6 | ||
| No | 76 | 88.4 | ||
| Nasal flaring | ||||
| Yes | 47 | 54.7 | ||
| No | 39 | 45.3 | ||
| Hypoxia | ||||
| Present | 9 | 10.5 | ||
| Absent | 46 | 53.5 | ||
| Not tested | 31 | 36.0 | ||
| Tachypnea | ||||
| Yes | 68 | 79.1 | ||
| No | 18 | 20.9 | ||
| Crepitations | ||||
| Yes | 41 | 47.7 | ||
| No | 45 | 52.3 |
IQR: Interquartile range
Tachypnea was observed in more than half of the participants (68, 79.1%). Intercostal retraction, subcostal retraction, and lower chest wall retraction were observed in 68 (79.1%), 61 (70.9%), and 29 (33.7%) of the children, respectively. Furthermore, grunting and nasal flaring were observed in 10 (11.6%) and 47 (54.7%) of the children, respectively, while crepitations were detected in 41 (47.7%). Of the 55 patients who had their SpO2 checked on admission, 9 (16.4%) had hypoxemia [Table 2].
Out of 86 children with a clinical diagnosis of severe pneumonia, ultrasonography signs of pneumonia were detected in 68 (79.1%).
Table 3 showed that 55(64%) of the children had ultrasound features of consolidation with the highest frequency in the right anterior zone (29, 52.7%) followed by the right posterior (26, 47.3%). Florid B-lines were found in 29 (23.3%) of the LUS, while pleural fluid was seen in 20 (23.3%) children with equal frequencies of 10 (50%) on the right and left sides, respectively.
| Ultrasound findings | Frequency (%) |
|---|---|
| Lung consolidation | 55 (64.0) |
| Location of consolidation | |
| Right anterior | 29 (52.7) |
| Right posterior | 26 (47.3) |
| Right lateral | 20 (36.4) |
| Left anterior | 23 (41.8) |
| Left posterior | 24 (43.6) |
| Left lateral | 18 (32.7) |
| Interstitial edema/florid B-lines | 29 (33.7) |
| Pleural fluid | 20 (23.3) |
| Location of pleural fluid | |
| Right | 10 (50.0) |
| Left | 10 (50.0) |
Among the 68 children who had ultrasound diagnosis of pneumonia, 29 (42.6%) had lung consolidation only, 12 (17.6%) had both lung consolidation and interstitial edema, 7 (10.3%) had a combination of consolidation, interstitial edema, and pleural fluid while 3 (4.4%) had only pleural fluid [Figure 3].
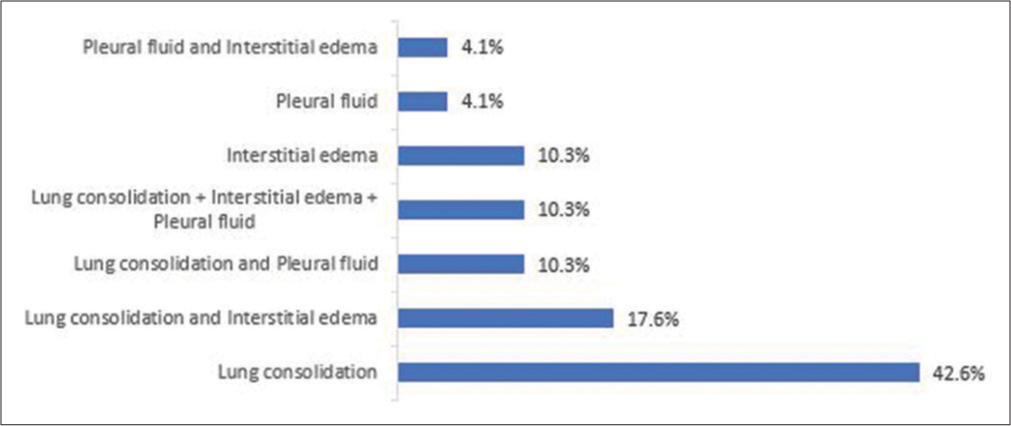
- Presentation of pneumonia on ultrasound among participants.
When the association between the clinical features and ultrasound diagnosis of pneumonia was tested, duration of cough and crepitation were the only features that showed significant association with the diagnosis of pneumonia on ultrasound (P < 0.05). A higher proportion of males who had pneumonia (88.0%) were identified on ultrasound compared to 66.7% of female children identified on ultrasound (P = 0.016). The median duration of cough of 5 days (interquartile range [IQR]: 2.50; 8.50) days) among children diagnosed with pneumonia on ultrasound was statistically significantly higher than the median duration of cough among children who did not have an ultrasound diagnosis of pneumonia (3 days, IQR: 1.50; 4.00) P = 0.046. Furthermore, a higher proportion of the children with crepitation (37, 90.2%) were diagnosed with pneumonia on ultrasound compared to the proportion of children without crepitation who were diagnosed with pneumonia on ultrasound (30, 68.9%), P = 0.015 [Table 4].
| Variables | Diagnosis of pneumonia in the US | P-value | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| Frequency (%) | Mean±SD or Median (IQR) | Frequency (%) | Mean±SD or Median (IQR) | ||
| History of cough | |||||
| Yes | 56 (83.6) | 11 (16.4) | 0.106 | ||
| No | 12 (63.2) | 7 (36.8) | |||
| Duration of cough (days) | 5.00 (2.50; 8.50) | 3.00 (1.50; 4.00) | 0.046 | ||
| History of fever | |||||
| Yes | 56 (76.7) | 17 (23.3) | 0.285 | ||
| No | 12 (92.3) | 1 (7.70) | |||
| Tachypnea | |||||
| Yes | 56 (82.4) | 12 (17.6) | 0.106 | ||
| No | 11 (61.1) | 7 (38.9) | |||
| Intercostal retraction | |||||
| Yes | 56 (82.4) | 12 (17.6) | 0.192 | ||
| No | 12 (66.7) | 6 (33.3) | |||
| Subcostal retraction | |||||
| Yes | 51 (83.6) | 10 (16.4) | 0.106 | ||
| No | 17 (68.0) | 8 (32.0) | |||
| Lower chest wall retraction | |||||
| Yes | 24 (82.8) | 5 (17.2) | 0.549 | ||
| No | 44 (77.2) | 13 (22.8) | |||
| Grunting | |||||
| Yes | 9 (90.0) | 1 (10.0) | 0.681 | ||
| No | 59 (77.6) | 17 (22.4) | |||
| Nasal flaring | |||||
| Yes | 39 (83.0) | 8 (17.0) | 0.328 | ||
| No | 29 (74.4) | 10 (25.6) | |||
| Crepitations | |||||
| Yes | 37 (90.2) | 4 (9.8) | 0.015 | ||
| No | 31 (68.9) | 14 (31.1) | |||
SD: Standard deviation, IQR: Interquartile range
DISCUSSION
In LMICs, CAP is the leading cause of death in the under-5 populations; therefore, early identification of severe cases is important to help ensure prompt treatment aiming to reduce mortality.[4,5] According to the WHO, the initial diagnosis of CAP can be based on some clinical features, which include cough, fever, tachypnea, increasingly labored breathing, rhonchi, crepitations, and wheezing. However, tachypnea has been identified as the most significant clinical sign in the presence of a cough.[17,20] In this study, the most frequent constitutional symptom was fever, which was present in more than three-quarters (84.9%) of the patients, while cough was documented in 77.9%. Tachypnea and intercostal retraction were the most common clinical signs seen in 79.1% of the participants, respectively, followed closely by subcoastal retraction and nasal flaring. The least frequent clinical signs were grunting and hypoxia. These clinical features agreed with the findings by Abdulkarim et al.[21] in Ilorin, Nigeria, who also documented fever as the commonest symptom; however, crepitation was the commonest sign found in 92.2%, while tachypnea was found in 85.6%. However, in concordance with our study, grunting and cyanosis were the least frequent clinical signs. Karim et al.[20] from Hayatabad also had tachypnea as the most common clinical sign, followed by crepitation, although, the frequencies were higher than those found in our study, accounting for 91.4% and 75%, respectively. Noisy breathing was, however the most frequent presenting complaint in their study. These varying findings might be due to the different age ranges of the population studied.
In the present study, the age group that was most frequently diagnosed with clinical pneumonia was the 2–12 months age group, followed by the 13–24 months age group. This was in concordance with the finding by Karim et al.[20] whose most common age group was 2–23 months. Many studies done among the pediatric population[4,10] used 16 years of age as the upper limit for the pediatric study age groups, but their mean ages still fell under five years in most of the studies. This is because the burden of CAP is highest among children under five years, and they tend to come down with the severe form when compared with the older age groups. The reduced level of immunity in children, when compared to adults, may be the reason for this.
Many studies[4,11,22] reported a male preponderance in CAP, and this is in agreement with the findings from this study. The theory proposed for this was the difference in the circulating hormones in both genders, but this theory was disputed by others, making the male preponderance unexplainable. In contrast, the study by Karim et al.[20] found female preponderance, while Orimadegun and Myer.[23] a study conducted in six centers in Nigeria found no statistically significant difference in the prevalence of pneumonia in relation to sex. However, in the same study by Orimadegun and Myer[23], when the children’s weights were used, there was a male predominance among the underweight children, but no definite reason was found for this.
For some time, there has been the concept that ultrasound could not be employed for the evaluation of the lung. This is due to the presence of air, which causes a high acoustic mismatch with the surrounding tissues and results in complete reflection of the ultrasound beam, thereby preventing direct imaging of the lung parenchyma.[15] Research has, however, shown that distortion of the normal artifacts seen in the lung, for example, A-lines, B-lines, and pleural lines, can be used to diagnose some lung conditions, including pneumonia.[12-18] Features that have been identified as evidence of pneumonia on ultrasound include the replacement of the normal A- and B-lines with a hypoechoic structure that looks like the liver in the subpleural regions, with echogenic lines seen within it consistent with air bronchogram, which represents an area of consolidation.[12-18] Other features include confluent or florid B-lines, which is termed interstitial phenomenon, and pleural fluid collection. Some studies described consolidations as either pleural line abnormality, shred line, fluid bronchograms, superficial fluid alveologram, or vascular tree-shaped pattern (Doppler mode).[24,25]
There is currently no agreement on the sonographic criteria for the diagnosis of pneumonia in children.[16] While some studies made the diagnosis based on the presence of consolidation alone,[11,18,26,27] others based their diagnosis on the presence of either consolidation, B-line artifacts (interstitial phenomenon), or pleural fluid.[11,13,24,25] The presence of one or more of the features was used to diagnose pneumonia in these studies, and this was the criteria used in the present study.
Consolidation was seen on ultrasound in 64% of patients in our study, with the highest frequency in the right anterior zone. Florid B-lines were seen in 33% while pleural effusion was seen in 23.3% of the participants. These findings were slightly higher when compared with findings by Ianniello et al.[28] with 56%, 24%, and 18% having consolidation, florid B-lines, and pleural effusion, respectively. Results from Çağlar et al.[29] in their study in Turkey, among 91 children, were also lower, with consolidation found on ultrasonography in 51 (56%) children, florid B-lines in 14 (15.4%), and pleural fluid found in 9 (9.9%) of the participants. Iorio et al.[10] in their study found consolidation, in 26 (50%) of the 52 children evaluated. Their low percentage may be because they included both mild and severe cases in the study.
Claes et al.[30] their study used only consolidation in the diagnosis of pneumonia and, in addition, measured the area of consolidation and number seen per patient. Some recent studies[31-33] have shown that the size and number of areas of consolidation on ultrasound can be used to determine the etiology of pneumonia confidently. They found out that larger consolidations were linked with bacterial origin while multiple small consolidations, usually <10 mm, are diagnostic of viral or atypical pneumonia. This was, however not tested in the index study.
The capability of lung ultrasound to diagnose pneumonia in children with clinical features suspicious of pneumonia has significantly improved with more experience in the act. Ho et al.[19] and Ahmad et al.[22] had ultrasound diagnosis in 97.5% and 96.8% of children with clinical diagnoses, which were significantly higher than the 79.1% obtained from this study. Çağlar et al.[29] and Ianniello et al.[28] on the contrary, recorded slightly lower percentages of 61.5% and 71.4%, respectively. The variations in the percentages could be attributed to the expertise of the sonologists/sonographers.
This was confirmed in the study by Shah et al.[34] where the ultrasound diagnosis was 24%, and the reason given was their use of multiple operators with varied ultrasonography experiences.
When the association between the clinical features and ultrasound diagnosis of pneumonia was tested in the present study, the features that showed significant association were duration of cough and crepitation. This was contrary to the finding by Karim et al.,[20], in which bronchial breath sound and tachypnea were the most significant association with radiological findings. Furthermore, Berce et al.[31] found bronchial breath sound and chest pain as the clinical features with significant association. The variance in the significant clinical features may be due to the different age groups studied.
The limitations of this study include the fact that few numbers of children were recruited due to the restrictions of admissions into the children’s emergency ward during the study period due to COVID-19. Furthermore, the accuracy of LUS in the diagnosis was not determined because the lung ultrasound findings were not compared with other imaging modalities or laboratory diagnoses and there was only one pediatric radiologist scanned all the patients.
CONCLUSION
This study shows that LUS has a high capability to diagnose CAP in children with clinical suspicion, and it can be safely used at the point of care due to its lack of ionizing radiation and portability. It is therefore recommended that LUS could be used as one of the imaging modalities in the management of children with clinical suspicion of CAP in the tropics.
Acknowledgment
The authors wish to acknowledge all the resident doctors in the Department of Pediatrics of the University College Hospital, Ibadan, who rotated through the Children Emergency Ward during the study period.
Ethical approval
The research/study was approved by the Institutional Review Board at the Joint Institution Review Committee (IRC) of the University of Ibadan and University College Hospital, Ibadan, number UI/EC/20/0373, dated August 31, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This research was funded by the National Institute for Health Research (NIHR) (IMPALA, grant reference 16/136/35) using UK aid from the UK Government to support global health research.
References
- Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet. 2015;385:430-40.
- [CrossRef] [PubMed] [Google Scholar]
- Media center fact sheets on pneumonia. Available from: https://www.who.int/mediacentre/factsheets/fs331/en [Last accessed on 2022 Nov 19]
- [Google Scholar]
- The Burden and risks of pediatric pneumonia in Nigeria: A desk-based review of existing literature and data. Pediatr Pulmonol. 2020;55(Suppl 1):S10-21.
- [CrossRef] [Google Scholar]
- National and regional under-5 mortality rate by economic status for low-income and middle-income countries: A systematic assessment. Lancet Glob Health. 2018;6:e535-47.
- [CrossRef] [PubMed] [Google Scholar]
- Management of community acquired pneumonia (CAP) in children: Clinical practice guidelines by the paediatrics association of Nigeria (PAN) Niger J Paediatr. 2015;42:283-92.
- [CrossRef] [Google Scholar]
- Use of simple clinical signs to predict pneumonia in young Gambian children: The influence of malnutrition. Bull World Health Organ. 1995;73:299-304.
- [Google Scholar]
- Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408-16.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: A systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-31.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasound in the diagnosis of pneumonia in children: Proposal for a new diagnostic algorithm. PeerJ. 2015;3:e1374.
- [CrossRef] [PubMed] [Google Scholar]
- Can lung ultrasound replace chest radiography for the diagnosis of pneumonia in hospitalized children? Respiration. 2014;88:112-5.
- [CrossRef] [PubMed] [Google Scholar]
- The air bronchogram: Sonographic demonstration. AJR Am J Roentgenol. 1986;147:593-5.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest. 2014;146:383-8.
- [CrossRef] [PubMed] [Google Scholar]
- The value of bedside lung ultrasonography in diagnosis of neonatal pneumonia. Egypt J Radiol Nucl Med. 2013;44:339-47.
- [CrossRef] [Google Scholar]
- Lung ultrasound in evaluation of pneumonia. J Ultrasound Med. 2012;31:823-6.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of chest x-ray and lung ultrasound in the management of infants and children presenting with severe pneumonia in low-and middle-income countries: A pragmatic scoping review. J Glob Health. 2022;12:10013.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated management of childhood illness. 2014. Chart booklet. Available from: https://apps.who.int/iris/bitstream/10665/104772/16/9789241506823_chartbook_eng.pdf [Last accessed on 2022 Nov 19]
- [Google Scholar]
- Ultrasound diagnosis of pneumonia in children. Radiol Med. 2008;113:190-8.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of lung ultrasound in the diagnosis of community-acquired pneumonia in children. Pediatr Neonatol. 2015;56:40-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical findings and radiological evaluation of WHO-defined severe pneumonia among hospitalized children. Cureus. 2023;15:e33804.
- [CrossRef] [Google Scholar]
- Childhood pneumonia at the university of ilorin teaching Hospital. Ilorin Nigeria Niger J Paediatr. 2013;40:284-9.
- [Google Scholar]
- Diagnostic accuracy of lung ultrasound in diagnosis of pediatric pneumonia. Med Forum Mon. 2019;30:20-3.
- [Google Scholar]
- Sex-specific prevalence and trends in acute respiratory infection episodes among children less than 5 years in Nigeria. Niger J Clin Pract. 2019;22:1590-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic diagnosis and follow-up of pneumonia: A prospective study. Respiration. 2007;74:537-47.
- [CrossRef] [PubMed] [Google Scholar]
- International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577-91.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound detection of pneumonia in febrile children with respiratory distress: A prospective study. Eur J Pediatr. 2016;175:163-70.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasound for the diagnosis of pneumonia in children with acute bronchiolitis. BMC Pulm Med. 2018;18:191.
- [CrossRef] [PubMed] [Google Scholar]
- First-line diagnosis of paediatric pneumonia in emergency: Lung ultrasound (LUS) in addition to chest-X-ray (CXR) and its role in follow-up. Br J Radiol. 2016;89:20150998.
- [CrossRef] [PubMed] [Google Scholar]
- Is lung ultrasonography a useful method to diagnose children with community-acquired pneumonia in emergency settings? Hong Kong J Emerg Med. 2019;26:91-7.
- [CrossRef] [Google Scholar]
- Performance of chest ultrasound in pediatric pneumonia. Eur J Radiol. 2017;88:82-7.
- [CrossRef] [PubMed] [Google Scholar]
- The usefulness of lung ultrasound for the aetiological diagnosis of community-acquired pneumonia in children. Sci Rep. 2019;9:17957.
- [CrossRef] [PubMed] [Google Scholar]
- Can lung ultrasound differentiate between bacterial and viral pneumonia in children? J Clin Ultrasound. 2021;49:91-100.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasonography beyond the diagnosis of pediatrics pneumonia. Cureus. 2022;14:e22460.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013;167:119-25.
- [CrossRef] [PubMed] [Google Scholar]







