Translate this page into:
Pulmonary function assessments and clinical correlates in children with sickle cell disease in Cape Town, South Africa
*Corresponding author: Sandra Kwarteng Owusu Department of Child Health, Komfo Anokye Teaching Hospital, School of Medicine and Dentistry Kwame Nkrumah University of Science and Technology Kumasi Ashanti Region Ghana. abenaboamah18@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kwarteng Owusu S, Mapani MK, Visagie A, Marozva N, Yassin A, Vanker A, et al. Pulmonary function assessments and clinical correlates in children with sickle cell disease in Cape Town, South Africa. J Pan Afr Thorac Soc. 2024;5:33-44. doi: 10.25259/JPATS_30_2023
Abstract
Objectives:
Among children with sickle cell disease (SCD) in Africa, there are varied reports on pulmonary function assessments. Restrictive pulmonary function is common in children with SCD in Africa; however, reports from Africa are few. We aimed to describe pulmonary function and its clinical correlates in children with SCD in Cape Town, South Africa.
Materials and Methods:
A prospective cross-sectional study was carried out over seven months from October 2018 to April 2019 in children 6–16 years with SCD. Children with hemoglobin (Hb) genotypes, homozygous for the BS globin mutation, and sickle-beta0-thalassemia Hb were included in the study. Children were excluded if they had acute complications. Medical record review clinical, laboratory, and pulmonary function assessments were done. Data were entered into Excel and exported to Stata Version 16.0 statistical software for analysis.
Results:
A total of 25 participants were recruited, mean (standard deviation) age of 10 ± (3.0) years. Thirteen (53%) children were under ten years and 15 (60%) were male. The median/interquartile range age at diagnosis was 1.7 [0.8–3.0] years. SCD-related complications were common. A review of the medical records showed a third of the patients (32%) had at least one previous episode of acute chest syndrome, 20 (80%) had a history of vaso-occlusive crisis, and 15 (76%) had required at least one blood transfusion. Spirometry was performed on 19 (76%) of the participants 9 (47%) had abnormal lung function. The most common spirometry abnormality was a restrictive pattern (forced vital capacity (FVC) < lower limit of normal (LLN)). No participant had a positive bronchodilator response. Older age was associated with a decrease in forced expiratory volume in the first second (FEV1) Z-score (−0.16, 95% confidence interval [CI] −0.31, −0.01; P = 0.04). Children on hydroxyurea similarly had reduced FEV1 Z-score (−1.5, 95%CI −2.88, −0.12; P = 0.04) and reduced FVC Z-score (−2.21, 95%CI −3.64, −0.79; P < 0.001).
Conclusion:
Lung function abnormalities were common among children with SCD, with restrictive abnormality predominating. Asthma and obstructive airway abnormalities were uncommon in children with SCD in South Africa.
Keywords
Sickle cell disease
Children
Pulmonary function
Restrictive lung disease
INTRODUCTION
Sickle cell disease (SCD) is a severe life-limiting genetic disorder of blood with significant public health importance worldwide. SCD is highly prevalent among populations of African ancestry.[1-3] In sub-Saharan Africa, the prevalence of adults with heterozygous hemoglobin S ranges from 5% to 40% and >300,000 infants are born with sickle cell anemia in Africa annually.[1,3] Although the incidence of SCD in South Africa is low, it has been reported as an emerging health problem with a rising burden in Cape Town, South Africa, due to continuous migration of people from neighboring high-burden countries.[4]
Pulmonary complications are a leading cause of morbidity and mortality in SCD.[5-7] Among adults with SCD, pulmonary complications contribute to up to 20% of mortality.[8] The pathogenesis of pulmonary disease in SCD is likely driven by abnormal interaction between erythrocytes, leucocytes, platelets, and vascular endothelium resulting from episodes of hypoxia leading to vaso-occlusion and impaired microvascular blood flow. In addition, episodes of tissue ischemic reperfusion injury and hemolysis promote inflammation, thrombosis, and oxidative stress.[9]
The pulmonary disease burden in SCD leads to chronic complications affecting the airways, parenchyma, and vasculature, reflected by impairment in lung function.[10]
Among patients with SCD, pulmonary function abnormalities are reliable as a first sign to help early diagnosis of chronic lung disease. Pulmonary function tests commonly used in assessing the presence of lung disease in sickle cell include spirometry, single breath diffusion capacity of the lung for carbon monoxide (DLCO), oscillometry (OSC), and 6-minute walk test (6-MWT).[10-14]
DLCO measures alveolar volume (AV) and derives the carbon monoxide transfer coefficient (KCO) useful to detect the extent of alveolar damage from vascular pathology in SCD.[15] Spirometry measures dynamic lung volumes and detects airway normality, distinguishes restrictive from obstructive or mixed patterns, and can inform the use of inhaled corticosteroids in those with airway hyper-reactivity.[16] OSC measures the mechanical properties of the respiratory system in tidal breathing and documents respiratory system resistance, a measure of airway caliber, and respiratory system reactance, a measure of the stiffness of the chest wall, lungs, and airways.[17]
6-MWT is a cardiopulmonary assessment; it measures the distance a patient can walk in 6 min, oxygen saturation and perception of dyspnea during exertion.[18]
In patients with SCD spirometry assessment, reports range from normal, obstructive, restrictive, mixed, or non-specific patterns in children and adolescents with SCD.[10-14] Among a cohort of 149 children and adolescents with SCD in the UK, abnormal spirometry was found in 45 (30%) of them: 16% obstructive, 7% restrictive, 1% mixed, and 6% non-specific patterns.[11]
Further, DLCO and OSC measurements were also significantly impaired in the same cohort of children with SCD.[10]
Outside Africa, pulmonary function assessments in children with SCD show a high prevalence of asthma as a common comorbid condition, with prevalence ranging from 17% to 28% in young children with SCD.[11,19,20] Similarly, a high prevalence of lower airway obstruction and airway hyper-reactivity has been reported in children with SCD, predominantly from Europe and North America, 57% and 77%, respectively. The inflammatory process that promotes reversible and fixed airway obstruction in SCD is suggested to be complex, including both allergic and non-allergic pathways.
Despite the high prevalence of SCD in Africa, there have been few reports on pulmonary function assessments in African children with SCD. Furthermore, there is also limited data on the contribution of early initiation of disease-modifying agents such as hydroxyurea (HU) on pulmonary function assessments, as the use of HU is limited in Africa.[13,14,21-23] In addition, data on the clinical correlation between disease-related complications and pulmonary function measurements in African children affected with SCD are also limited as there are no longitudinal studies. This study aimed to describe pulmonary function assessments using Spirometry, single breath DLCO, and OSC and its clinical correlates in children living with SCD in Cape Town, South Africa.
MATERIALS AND METHODS
Study design
A prospective cross-sectional study was carried out over seven months from October 2018 to April 2019 in children with SCD who attend outpatient clinics at the Red Cross War Memorial Children’s Hospital in Cape Town, South Africa. This is a public tertiary hospital that receives referrals from across all of the Western and Eastern Cape provinces and neighboring countries such as Malawi and Angola. The clinic is attended by children from South Africa and the neighbouring countries. Children in the SCD clinic who met the inclusion criteria were consecutively recruited weekly during follow-up clinics for children with SCD. Written informed consent was obtained from a parent or legal guardian, and assent was obtained for children above seven years. Ethical approval was obtained from the Human Research Ethics Committee of the University of Cape Town (HREC 345/2018).
Inclusion criteria
Children aged 6–16 years with SCD based on the presence of any of the following: Hemoglobin (Hb) genotypes, HbSS (homozygous for the BS globin mutation) or HbSC (heterozygous for the BS-globin mutation) or sickle-beta0-thalassemia Hb; and who were clinically stable.
Exclusion criteria
Children with acute complications at the time of screening, including acute chest syndrome (ACS), lower respiratory tract infection (LRTI), vaso-occlusive crisis (VOC), hemolytic crisis or pneumonia, as well as those with any neurologic impairment. Children were also excluded if they had a recent history of any acute episode and were considered not to have completely recovered.
Data collection
Basic sociodemographic information, immunization, medication history, and details of previous acute events, such as previous blood transfusions, anemia, and episodes of ACS and LRTI, were captured from medical records.
Clinical information
History of respiratory symptoms in the past 12 months, including chest tightness, wheezing, shortness of breath after exercise, cough frequency, and details of previous hospital visits for respiratory reasons were collected. The use of any asthma medications prescribed by a doctor and doctor-diagnosed asthma was also recorded by questioning and medical records review. These questions were based on the International Society of Asthma and Allergies in Childhood-validated questionnaires.[24] Clinical assessments, including anthropometric measurements and respiratory examination, were carried out. Each participant had blood taken for hemoglobin and white cell count. A chest X-ray was performed for each participant and reported by two pulmonologists. Where there was disagreement a third pulmonologist reported to help resolve the disagreement.
Lung function measurements
Spirometry was performed for children 6–16 years based on American Thoracic Society and European Respiratory Society guidelines.[12] Care fusion Jaeger Spiro VyntusÔ was used for all measurements. Forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, and forced expiratory flow between 25% and 75% of FVC (FEF25–75) were collected after three reproducible measurements were collected. Post-bronchodilator measurements were done after administration of 400 mg of salbutamol metered dose inhaler with a spacer. A difference in FEV1 or FVC post bronchodilator of ≥12% was considered a positive bronchodilator response (BDR).[13] Based on the recorded values from spirometry, FEV1 and FVC Z-scores were derived using the Global Lung Initiative (GLI) standardized tables.[25] The lower limit of normal (LLN) for spirometry was established at −1.64 Z score (5th percentile). We classified patterns as normal FEV1 and FVC ≥ LLN; obstructive as FEV1/FVC < LLN; restrictive FVC< LLN; and mixed as FEV1/FVC and FVC < LLN.[26,27]
OSC measurements were collected before spirometry using the Airwave Oscillometry Device tremoflo® C-100 (Thorasys, Montreal, Canada). OSC measurements were carried out for a minimum of three 16-s epochs based on international consensus guidelines.[14] The Z-scores for respiratory system resistance at 5 Hertz (R5) and respiratory system reactance at 5 Hertz (X5) were derived.
Single-breath DLCO was also carried out for children with a vital capacity of at least a liter, following international guidelines. Abnormal DLCO was based on GLI standard interpretation of Z-score < −1.64.[15]
Each participant undertook a 6-MWT to assess cardiopulmonary function, and we recorded the oxygen saturation pre- and post-activity, and the distance walked in meters.[18]
Statistics
All data were entered into a RedCapÔ database (Vanderbilt University, Nashville, Tennessee, United States of America). Data were analyzed with Stata SE Version 17.0 software Stata Corp Texas USA. Descriptive summary statistics of explanatory (patient characteristics) and outcome characteristics of the study participants were done. Mean and standard deviation (SD) were used to describe continuous variables that were normally distributed [Table 1]. In addition, variables that were not normally distributed were expressed as median and interquartile range (IQR) [Table 1]. Proportions were provided for the categorical variables [Table 1]. Mean and SD were provided for the pulmonary function measurement outcome variables: spirometry- FEV1, FVC, and Z scores; (FEV1 Z-score and FVC Z-score), OSC- R5, X5, R5 Z-score, X5 Z-score, DLCO- DLCO and DLCO Z-score and distance covered during the 6-MWT, as well as the pre-and post-exertion oxygen saturation were provided [Table 2].
| Participant characteristics | n(%) n=25 |
|---|---|
| Age in years mean (SD) | 10.0 (3.0) |
| Male | 15 (60) |
| Age of child at diagnosis, n=24┴mean (SD) | 2.6 (2.9) |
| BMI at enrolment n=22 | |
| Normal | 18 (81.8) |
| Underweight | 4 (18.2) |
| Clinical characteristics | |
| Diagnosed with asthma | 2 (8.0) |
| Wheezing after exercise | 6 (24.0) |
| Repetitive episodes of wheezing | 2 (8.0) |
| Episodes of wheezing in the past 12 months | 4 (16.0) |
| Sleep interrupted by wheezing | 2 (8.0) |
| Child ever prescribed asthma medication | 3 (12.0) |
| Emergency room visited for wheezing | 1 (4.0) |
| Hospital admission for wheezing | 2 (8.0) |
| Hemoglobin Hb SS Genotype* | 24 (96.0) |
| Treatment | |
| Penicillin prophylaxis | 21 (84.0) |
| Hydroxyurea therapy | 22 (88.0) |
| Median (IQR) duration of hydroxyurea therapy year | 2.8 (1.4–7.0) |
| Disease-related complications | |
| Previous hospitalization related to SCD | 20 (80.0) |
| Previous hemotransfusion | 19 (76.0) |
| One or more episodes of anemia | 16 (64.00) |
| Previous hospitalization for LRTI | 9 (36.00) |
| Previous hospitalization for VOC | 20 (80.00) |
| Previous admission for acute chest syndrome | 8 (32.00) |
| Environmental exposures | |
| Environmental tobacco smoke exposure – | 3 (12.0) |
| Hemoglobin level | |
| Hb (g/dL) (n=21) – hemoglobin level | 7.8 (7.0–8.9) |
| Chest X-ray n=25 | |
| Normal chest X-ray | 3 (12) |
| Any abnormality on chest X-rays | 22 (80) |
| Bronchiectasis | 2 (9.09) |
| Increased broncho vascular markings | 11 (44) |
| Reticular pattern | 1 (4) |
| Air trapping | 4 (16) |
| Cardiomegaly | 3 (12) |
| Ground glass appearance | 1 (4) |
LRTI: Lower respiratory tract infections, VOC: Vaso-occlusive crisis, SCD: Sickle cell disease, SD: Standard deviation. IQR: Interquartile range (g/dL) Gram per decilitre, WCC: White cell counts, Hb: Hemoglobin. *One participant had haemoglobin Sβthal┴ Age at diagnosis was missing for one participant, BMI: Body mass index
| Spirometry (n=19) | |
| FEV (L) mean (SD) | 1.50 (0.4) |
| FEV1 Z scores | −1.48 (1.0) |
| Z-score below−1.64, n(%) | 9 (47) |
| FVC (L) mean (SD) | 1.7 (0.5) |
| FVC (L) Z-score mean (SD) | −1.7 (1.1) |
| Z -score below−1.64 n(%) | 9 (47) |
| FEF 25–75 mean (SD) | 2.0 (0.5) |
| FEF25–75 Z-score | −0.6 (0.9) |
| Z-score below−1.64, n(%) | 2 (11) |
| FEV/FVC mean (SD) | 0.9 (0.1) |
| FEV/FVC Z-score | 0.4 (1.0) |
| FEV/FVC Z-score <1.64 | - |
| FEV1 BD % change >12% | - |
| Oscillometry (n=12) | |
| R5 (cmH2O.s/L) | 6.5 (1.5) |
| Z- score below−1.64, n(%) | 2 (16.6) |
| X5 (cmH2O.s/L) | −3.0 (0.99) |
| Z-score below−1.64, n(%) | 0 |
| Diffusion capacity (n=8) | 13.5 (3.1) |
| DLCO range | 9.5–19.8 |
| DLCO Z-score | −1.6 (0.7) |
| DLCO Z-score below−1.64 n(%) | 3 (37) |
| Six-minute walk test (n=18) | |
| Distance walked in meters | 548 (108.4) |
| Range of distance walked | 408–708 |
| Desaturation <90% during test, n(%)- | 6 (33.3) |
| Oxygen saturation pre-activity mean (SD) | 97.1 (2.31) |
| Oxygen saturation post activity mean (SD) | 88.9 (8.19) |
All values are represented in mean and SD unless otherwise stated, n: Number and %: Percent. FEV1: Forced expiratory volume in the first second, FVC: Forced vital capacity, FEF25–75: Forced expiratory flow between 25% and 75% of FVC, R5: Resistance at 5Hz, X5 Reactance at 5Hz, R5–20: Difference between resistance at 5 and 20 Hz, AX: Area under the reactance curve, DLCO: Diffusion capacity of the lung for carbon monoxide, LLN: Lower limit of normal, SD: Standard deviation, OSC: Oscillometry, BDR: Bronchodilator response, MWT: Minute walk test
Lung function outcome variables FEV1, FVC, FEF25–75, FEV1/FVC, and DLCO measurements R5 and X5 expressed as Z-scores were modeled using both univariate and multivariate linear regression to assess the impact of different patient characteristics on lung function. Multivariate models were adjusted for a set of confounders identified through previous literature and univariate associations in the data. The confounders included age, age at diagnosis, sex, HU therapy, previous hospitalization, previous blood transfusion, previous ACS, previous LRTI, previous VOC, chest X-ray findings, and wheezing in the past 12 months.
A significance level of 5% was used to guide the interpretation of all P-values.
RESULTS
Demographic, clinical characteristics, and laboratory parameters
A total of 25 participants were recruited, mean (SD) age of 10.0 (3.0) years [Table 1]. Thirteen 13 (53.0%) children were under 10 years. There was a male predominance, 15 (60%). The median (IQR) age at diagnosis was 1.7 (0.8 3.0) years. There were four participants who were underweight 4 (18.2%). The majority of children 22 (88.0%) were on HU therapy with a median (IQR) duration of 2.8 (1.4–7.0) years [Table 1].
SCD-related complications were common. A third of the patients (32%) had at least one episode of ACS, 80% a history of VOC, and 76% had required at least one blood transfusion [Table 1]. In addition, respiratory disease was commonly reported with 25% having had a history of LRTI, 24% reported wheezing episodes in the past, with 16% having an episode of wheezing in the previous 12 months, including two children requiring hospitalization following an episode of wheezing. However, asthma diagnosis and treatment were uncommon in this cohort: 2 (8.0%) had been diagnosed with asthma by a doctor and 3 (12%) had been prescribed asthma medications in the past year [Table 1]. The median hemoglobin was 7.8g/dL (IQR 7.0–8.9) and white cell count, 11.0 × 10/L, (8.1–12.9) and eosinophil percentage was low with a median value of 2.0 (1.0–5.7%) [Table 1].
Most participants, 22 (88%), had abnormal chest X-rays with the predominant abnormality being the presence of increased bronchovascular markings [Table 1]. Agreement between the two reporters on the presence of any abnormality on the chest X-rays was good, kappa 0.5–1.0 [Table S1].
Lung function
Spirometry was performed in 19 (76%), oscillometry in 13 (52%), DLCO in 8 (32%), and 6 min walk in 18 (48%) participants. Lung function outcomes are summarized in Table 2. Nearly, half of the children who underwent spirometry 9 (47%) had low spirometric lung function, and FEV1 <LLN and/or FVC <LLN [Table 2 and Figure 1]. The commonest spirometry abnormality was a restrictive pattern. No participant had a positive BDR [Table 2 and Figure 1]. Two of the children who underwent OSC 2 (16.6%) participants had low R5. Three of the children who underwent DLCO 3 (37.5%), had DLCO <LLN. Six of the 12 participants who underwent 6- MWT 6 (33.4%) desaturated to <90% with 6 min of activity and the average distance walked was nearly 550 m [Table 2].
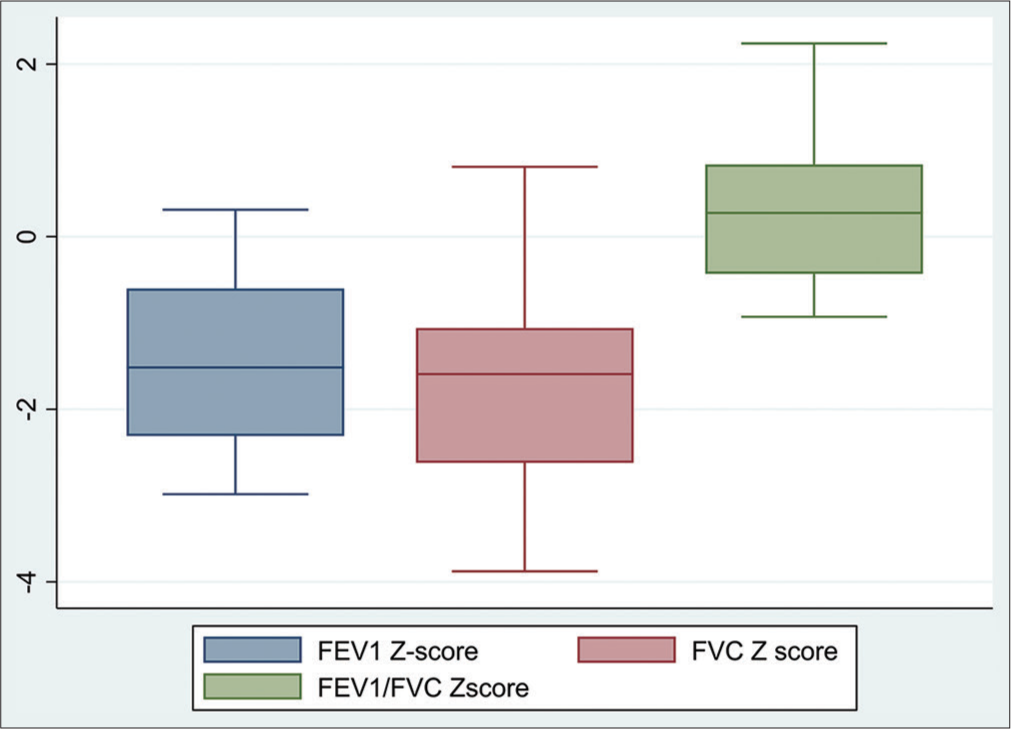
- Box plot comparative analysis of the Z-score for forced expiratory volume in the first second (FEV1), Z-score forced vital capacity (FVC), and ratio FEV1/FVC Z-scores of patients with sickle cell disease.
Older age was associated with a decrease in FEV1 Z-score (−0.16, 95% confidence interval [CI] −0.31, −0.01; P = 0.04). Children on HU similarly had reduced FEV1 Z-score (−1.5, 95%CI −2.88, −0.12; P = 0.04) and reduced FVC Z-score (−2.21, 95%CI −3.64, −0.79; P < 0.001). The previous hospitalization was associated with increased FEV1 Z-score (−1.51, 95% CI 0.43, −2.59 P = 0.01) and FVC Z-score (−1.59, 95% CI 0.3, −2.89 P = 0.002). These changes were, however, no longer significant after adjusting for confounding factors. Clinical characteristics were not significantly associated with FEV1/FVC and FEF25–75 Z-scores [Tables 3 and 4].
| FVC Z-scores | FEV1Z-score | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted model* | Unadjusted model | Adjusted model* | |||||
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Age of child (years) | −0.15 (−0.33, 0.03) | 0.09 | −0.08 (−0.5, 0.33) | 0.6 | −0.16 (−0.31, −0.01) | 0.04** | −0.1 (−0.57, 0.37) | 0.57 |
| Age at diagnosis (years) | −0.03 (−0.18, 0.13) | 0.71 | −0.01 (−0.32, 0.29) | 0.92 | −0.03 (−0.18, 0.11) | 0.65 | −0.04 (−0.39, 0.31) | 0.79 |
| Sex: (female vs. male) | −0.44 (−1.55, 0.66) | 0.41 | −0.42 (−1.83, 1) | 0.46 | −0.27 (−1.25, 0.7) | 0.56 | −0.05 (−1.67, 1.56) | 0.93 |
| Hydroxyurea therapy (yes vs. no) | −2.21 (−3.64, −0.79) | <0.001** | −0.59 (−1.9, 0.71) | 0.24 | −1.5 (−2.88, −0.12) | 0.04** | 1.92 (−6.56, 10.4) | 0.52 |
| Previous hospitalization (yes vs. no) |
1.59 (0.3, 2.89) | 0.02** | 0.83 (−2.38, 4.05) | 0.51 | 1.51 (0.43, 2.59) | 0.01 | 0.96 (−2.71, 4.63) | 0.51 |
| Previous blood transfusion: (yes vs. no) |
1.06 (−0.19, 2.32) | 0.09 | 0.8 (−1.76, 3.36) | 0.44 | 0.97 (−0.11, 2.05) | 0.07 | 0.61 (−2.31, 3.53) | 0.59 |
| Previous LRTI episodes: (yes vs. no) |
−0.42 (−1.56, 0.72) | 0.45 | 0.86 (−0.71, 1.01) | 0.31 | −0.48 (−1.45, 0.5) | 0.31 | 0.66 (−3.75, 5.08) | 0.58 |
| Previous admission for vaso-occlusive crisis (yes vs. no) | 0.44 (−0.91, 1.79) | 0.5 | 1.83 (−1.68, 1.98) | 0.22 | 0.32 (−0.86, 1.5) | 0.57 | 0.51 (−1.84, 2.85) | 0.58 |
| Previous Admission for acute chest syndrome (yes vs. no) | 0.37 (−0.88, 1.62) | 0.54 | −0.18 (−2.05, 1.7) | 0.81 | 0.36 (−0.73, 1.44) | 0.5 | 1.42 (−4.07, 6.91) | 0.38 |
| Hemoglobin level u/L | 0.21 (−0.19, 0.61) | 0.28 | 0.43 (−0.03, 0.89) | 0.06 | 0.11 (−0.24, 0.46) | 0.53 | 0.22 (−0.3, 0.74) | 0.31 |
| Household smoke exposure (yes vs. no) |
−0.53 (−2.04, 0.97) | 0.46 | −0.44 (−2.3, 1.42) | 0.55 | −0.41 (−1.73, 0.9) | 0.51 | −0.42 (−2.54, 1.71) | 0.61 |
| CXR: Abnormal | 0.77 (−0.71, 2.25) | 0.29 | 0 (−2.47, 2.48) | 1 | 0.38 (−0.94, 1.69) | 0.55 | −0.38 (−3.2, 2.45) | 0.73 |
| Child wheeze past 12 months (yes vs. no) |
1.21 (−0.19, 2.61) | 0.09 | 0.65 (−1.62, 2.91) | 0.47 | 0.75 (−0.52, 2.02) | 0.23 | 0.25 (−2.33, 2.83) | 0.8 |
Analyses used linear regression and results are presented as coefficients and 95% CI, demographic characteristics and clinical parameters adjusted for in the model are listed in the table. *Model adjusted for age of child, age at diagnosis, sex, hydroxyurea therapy, previous hospitalization, previous hemotransfusion, previous LRTI episodes, previous admission for vaso-occlusive crisis, previous admission for acute chest syndrome, hemoglobin level, CXR, and child wheeze. FEV1: Forced expiratory volume in the first second, FVC: Forced vital capacity, SCD: Sickle cell disease, CI: Confidence interval, LRTI: Lower respiratory tract infection, CXR: Chest X-ray, **P< 0.05 was statistically significant.
| FEF25–75Z- score values | FEV1/FVC Z-score values | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted model* | Unadjusted model | Adjusted model* | |||||
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Age of child (years) | −0.12 (−0.27, 0.03) | 0.1 | 0.02 (−0.5, 0.55) | 0.91 | −0.12 (−0.27, 0.03) | 0.1 | 0.02 (−0.5, 0.55) | 0.91 |
| Age at diagnosis (years) | 0.01 (−0.14, 0.16) | 0.9 | −0.05 (−0.41, 0.31) | 0.74 | 0.01 (−0.14, 0.16) | 0.9 | −0.05 (−0.41, 0.31) | 0.74 |
| Sex: (female vs. male) | −0.04 (−0.99, 0.9) | 0.92 | 0.38 (−1.53, 2.29) | 0.63 | −0.04 (−0.99, 0.9) | 0.92 | 0.38 (−1.53, 2.29) | 0.63 |
| Hydroxyurea therapy (yes vs. no) |
0.28 (−1.22, 1.77) | 0.7 | 5.38 (−4.31, 15.07) | 0.18 | 0.28 (−1.22, 1.77) | 0.7 | 4.06 (−3.82, 11.95) | 0.20 |
| Previous hospitalization (yes vs. no) |
0.99 (−0.16, 2.14) | 0.09 | −0.16 (−6.71, 6.38) | 0.94 | 0.99 (−0.16, 2.14) | 0.09 | 2.05 (−2.4, 6.51) | 0.29 |
| Previous hemotransfusion: (yes vs. no) |
−0.15 (−1.28, 0.98) | 0.79 | −1.55 (−5.2, 2.1) | 0.32 | −0.15 (−1.28, 0.98) | 0.79 | −1.55 (−5.2, 2.1) | 0.32 |
| Previous LRTI episodes: (yes vs. no) |
−0.16 (−1.12, 0.8) | 0.73 | 0.12 (−2.85, 3.1) | 0.9 | −0.16 (−1.12, 0.8) | 0.73 | −0.31 (−2.74, 2.11) | 0.71 |
| Previous admission for vaso-occlusive crisis (yes vs. no) | −0.24 (−1.37, 0.88) | 0.65 | −0.04 (−2.97, 2.89) | 0.97 | −0.24 (−1.37, 0.88) | 0.65 | −0.04 (−2.97, 2.89) | 0.97 |
| Previous admission for acute chest syndrome (yes vs. no) | 0.24 (−0.89, 1.36) | 0.66 | −0.27 (−3.27, 2.73) | 0.79 | 0.24 (−0.89, 1.36) | 0.66 | 0.14 (−2.3, 2.58) | 0.87 |
| Hemoglobin level g/dL | −0.06 (−0.39, 0.27) | 0.72 | 0.44 (−0.88, 1.75) | 0.37 | −0.06 (−0.39, 0.27) | 0.72 | −0.17 (−0.82, 0.48) | 0.53 |
| Household smoke exposure (yes vs. no) |
0.17 (−1.09, 1.43) | 0.78 | −0.56 (−3.15, 2.03) | 0.6 | 0.17 (−1.09, 1.43) | 0.78 | −0.56 (−3.15, 2.03) | 0.6 |
| CXR: Abnormal | 0.52 (−0.71, 1.76) | 0.38 | −0.61 (−3.88, 2.65) | 0.65 | 0.52 (−0.71, 1.76) | 0.38 | −0.61 (−3.88, 2.65) | 0.65 |
| Child wheeze past 12 months (yes vs. no) |
0.71 (−0.5, 1.92) | 0.23 | 0.78 (−2.45, 4) | 0.56 | 0.71 (−0.5, 1.92) | 0.23 | 0.78 (−2.45, 4) | 0.56 |
Analyses used linear regression and results are presented as coefficients and 95% CI, demographic characteristics and clinical parameters adjusted for in the model are listed in the table. *Model adjusted for age of child, age at diagnosis, sex, hydroxyurea therapy, previous hospitalization, previous hemotransfusion, previous LRTI episodes, previous admission for vaso-occlusive crisis, previous admission for acute chest syndrome, hemoglobin level, CXR and child wheeze, FEV1: Forced expiratory volume in the first second, FVC: Forced vital capacity, FEF25–75: Forced expiratory flow between 25% and 75% of FVC, SCD: Sickle cell disease, CI: Confidence interval, LRTI: Lower respiratory tract infection, CXR: Chest X-ray
Older age (0.25, 95% CI 0.05–0.46; P = 0.02), age at diagnosis (0.54, 95% CI 0.16–0.92; P = 0.01), and presence of abnormality on chest X-ray (−1.81, 95% CI −3.41–0.2, P = 0.03) were significantly associated with increasing R5. Increasing age was also associated with decreased X5 (−0.25 95% CI 0.48–0.02, P = 0.02). The presence of abnormality on chest X-rays was associated with increased X5 (0.99, 95% CI 0.06–1.91, P = 0.04). However, these clinical characteristics were not significant after adjusting for confounding factors [Table 5].
| Resistance (cmH2O/s/L) Z score values | Reactance (hPa.s.L-1) Z-score values | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted model* | Unadjusted model | Adjusted model* | |||||
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Age of child (years) | 0.25 (0.05, 0.46) | 0.02** | 0.37 (−3.13, 3.87) | 0.41 | −0.02 (−0.17, 0.13) | 0.78 | −0.17 (−1.75, 1.41) | 0.41 |
| Age at diagnosis (years) | 0.54 (0.16, 0.92) | 0.01** | −3.14 (−39.37, 33.09) | 0.47 | −0.25 (−0.48, −0.02) | 0.04** | 1.41 (−14.93, 17.76) | 0.47 |
| Sex: (female vs. male) | 0.19 (−1.69, 2.06) | 0.83 | 0.86 (−11.35, 13.07) | 0.54 | 0.24 (−0.81, 1.29) | 0.62 | −0.04 (−5.55, 5.47) | 0.94 |
| Hydroxyurea therapy (yes vs. no) | 1.31 (−0.88, 3.5) | 0.21 | −1.82 (−20.44, 16.81) | 0.43 | −0.19 (−1.53, 1.15) | 0.76 | −0.31 (−8.63, 8.02) | 0.72 |
| Previous hospitalization (yes vs. no) |
−1.57 (−3.67, 0.53) | 0.13 | −7.09 (−83.64, 69.47) | 0.45 | −0.39 (−1.71, 0.92) | 0.52 | 2.14 (−32.4, 36.68) | 0.57 |
| Previous hemotransfusion (yes vs. no) |
0 (−2.05, 2.04) | 1 | −0.9 (−13.73, 11.92) | 0.53 | −0.21 (−1.36, 0.94) | 0.69 | 0.31 (−2.52, 3.13) | 0.69 |
| Prev LRTI episodes (yes vs. no) | −0.12 (−1.92, 1.67) | 0.88 | 0.43 (−12.78, 13.65) | 0.9 | 0.25 (−0.76, 1.25) | 0.6 | −2.24 (−10, 5.52) | 0.17 |
| Previous admission for vaso-occlusive crisis (yes vs. no) | −2.52 (−5.19, 0.14) | 0.06 | −14.63 (−160.9, 131.65) | 0.42 | 1.48 (0, 2.97) | 0.05** | 6.2 (−59.8, 72.19) | 0.44 |
| Previous admission for acute chest syndrome (yes vs. no) | −0.15 (−2.2, 1.89) | 0.87 | 2.79 (−19.3, 24.89) | 0.35 | −0.17 (−1.33, 0.98) | 0.74 | −1.09 (−11.06, 8.88) | 0.4 |
| Hemoglobin level u/L | 0.01 (−0.63, 0.66) | 0.96 | 0.06 (−3.63, 3.62) | 0.99 | −0.01 (−0.34, 0.32) | 0.95 | −0.06 (−1.69, 1.58) | 0.74 |
| CXR: Abnormal | −1.81 (−3.41, −0.2) | 0.03** | −1.43 (−8.05, 5.19) | 0.22 | 0.99 (0.06, 1.91) | 0.04** | 0.64 (−2.32, 3.6) | 0.62 |
| The child wheeze the past 12 months (yes vs. no) |
0.11 (−1.94, 2.15) | 0.91 | 3.19 (−37.58, 43.95) | 0.5 | −0.11 (−1.27, 1.05) | 0.84 | −1.87 (−20.26, 16.53) | 0.42 |
Analyses used linear regression, and results are presented as coefficients and 95% CI; demographic characteristics and clinical parameters adjusted for in the model are listed in the table. *Model adjusted for age of the child, age at diagnosis, sex, hydroxyurea therapy, previous hospitalisation, previous hemotransfusion, previous LRTI episodes, previous admission for a vaso-occlusive crisis, previous admission for acute chest syndrome, hemoglobin level, CXR, and Child wheeze. **P<0.05 indicates statistically significant associations. CI: Confidence interval, LRTI: Lower respiratory tract infection, CXR: Chest X-ray
The previous hospitalization was associated with decreased DLCO Z-score (1.06, 95% CI 0.05–2.07 P = 0.04) in the univariate analysis only [Table 6].
| Z score values n=9 | ||||
|---|---|---|---|---|
| Unadjusted model | Adjusted model* | |||
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Age of child (years) | −0.12 (−0.29, 0.04) | 0.12 | 0.04 (−2.12, 2.21) | 0.85 |
| Age at diagnosis (years) | −0.05 (−0.38, 0.28) | 0.72 | −0.01 (−2.57, 2.56) | 0.98 |
| Sex: (female vs. male) | −0.28 (−1.56, 1) | 0.61 | −0.06 (−8.63, 8.52) | 0.95 |
| Hydroxyurea therapy (yes vs. no) | −1.27 (−2.71, 0.17) | 0.07 | ||
| Previous hospitalization (yes vs. no) | 1.06 (0.05, 2.07) | 0.04*** | 0.99 (−11.12, 13.1) | 0.49 |
| Previous hemotransfusion (yes vs. no) | 1.32 (−0.08, 2.71) | 0.06 | - | - |
| Previous LRTI episodes (yes vs. no) | 1.32 (−0.08, 2.71) | 0.06 | - | - |
| Previous admission for vaso-occlusive crisis (yes vs. no) | −0.13 (−1.39, 1.13) | 0.81 | - | - |
| Previous admission for acute chest syndrome (yes vs. no) | 0.45 (−1.42, 2.31) | 0.58 | 0.4 (−11.64, 12.45) | 0.74 |
| Hemoglobin level u/L | −0.09 (−0.57, 0.39) | 0.67 | - | - |
| CXR: Abnormal | 1.27 (−0.17, 2.71) | 0.07 | - | - |
| Child wheeze the past 12 months (yes vs. no) | 0.79 (−0.44, 2.03) | 0.17 | - | - |
DISCUSSION
Pulmonary function abnormalities were common, occurring in 47% of children with SCD despite a high proportion of them being on HU. Restrictive spirometry pattern predominated, and no child had a positive BDR. However, lung function abnormalities were heterogeneous, with reduced diffusing capacity of the lungs for carbon monoxide, increased respiratory system resistance, and desaturation with exercise also found. The majority of participants were diagnosed with SCD in 3 years. Vaso-occlusive episodes, anemia, and respiratory disease (LRTI and episodes of wheezing) were common. Abnormal chest X-ray findings were present in most children. Older age and being on HU were associated with reduced lung function. However, this significance was lost after adjusting for confounders.
Restrictive impairment on spirometry was the predominant lung function abnormality found, consistent with other African, but not high-income country settings. Across Africa, restrictive impairment on spirometry has been found in cross-sectional studies in Malawi, Nigeria, and the Central African Republic.[12-14]
Episodes of ischemia and inflammation mainly drive the pathophysiology of SCD. These events can, however, be reduced by early access to HU through the reduction of sickling events at the microvascular level of circulation.[28,29] As this cohort did not benefit from newborn screening, they would only have been diagnosed in childhood when already presenting with symptoms. In addition, the older children in this cohort would have been given HU based on severe disease as HU use was not routine at the time. It is likely that at the time of the introduction of HU, some of the children with severe symptoms may have suffered lung impairment already, leading to low lung pulmonary function that was noted. Thus, recurrent SCD-related insults are a possible cause of restrictive lung volumes. This may likely explain the predominant restrictive pattern seen in spirometry. Further, factors such as severe early-life LRTIs, which were common in our cohort, diagnosis of SCD following the occurrence of disease complications in our population, and possibly late introduction of disease-modifying agent, HU for severe disease may all have contributed to the development of small lung volumes in the children that we studied.[13,30]
Although wheezing episodes were common, none had evidence of obstructive lung function or positive BDR to support a diagnosis of asthma. Our findings agree with Cook et al. who reported that there was no evidence of positive BDR and obstructive airway disease in Malawian children.[12] In addition, other African studies by Arigliani et al. and Kuti and Adegoke showed a low prevalence of asthma among children with SCD, 7.1% and 3.8%, respectively.[13,14]
In contrast to our findings and the studies from Africa, studies from Europe suggest a predominance of airway hyper-reactivity and obstructive airway disease among children with SCD.[11,21,22] These differences may be attributed to age at diagnosis, with earlier diagnosis of SCD due to the existence of newborn screening programs and the early age at which the disease-modifying interventions are introduced in Europe. It is also likely that different environmental exposures may predispose to the development of airway hyperactivity and asthma in children with SCD living in Europe.
Abnormal oscillometry was noted in our participants; although the numbers were small, it is similar to what was reported by Lunt et al. among children with SCD in the UK. It has been proposed that increased resistance and reactance are due to compression of peripheral airways by engorged vessels.[10] In addition, low DLCO measurements were noted among some of the children assessed. Lunt et al., also reported reduced DLCO measurements among older children with SCD.[10] This possibly results from prior episodes of ischemia and decreased microvascular blood flow, causing lung injury through reduced AV.[31]
The occurrence of abnormal resistance and reactance measurements seen on oscillometery among our participants depicts occurrence of abnormal airway calibre and stiffened chest-wall in children with SCD. Similarly, abnormal DLCO measurements also show reduced AVs or damage due to progressing vascular pathology.
These potentially may contribute to chronic lung disease over time in SCD. Thus an important factor will be in the prevention or slowing down the progression of lung disease in children with SDC.
Our study found no relationship between the clinical characteristics of participants and pulmonary function on multivariate analysis, which may be related to our small sample size. Previous studies have reported a lung function decline with older age among children with SCD.[23] This was present in our unadjusted analysis and highlights the importance of early screening before school years with age-appropriate lung function methods to help identify children with pulmonary function abnormalities early to allow for follow-up of children with SCD.
The strength of this study is that this is a description of comprehensive lung function assessments among children with SCD in Cape Town, South Africa. Findings from this study will inform the need for consideration for the use of comprehensive lung function assessments to understand pulmonary disease in SCD better. The limitation of this study is the small number of children studied and the inclusion of children only from 6 years of age.
CONCLUSION
This study has shown the value of lung function assessments in delineating functional impairment in children with SCD. Lung function impairment is common and likely progressive even in well children on HU with SCD. Tools that can monitor lung function trajectory through early childhood are key so that pre-symptomatic disease can be identified and appropriate treatment, such as inhaled corticosteroids and bronchodilators instituted, to help reduce the future risk of poor lung health. Routine pulmonary function measurements will be an important tool to influence clinical decision-making about commencing HU therapy, the need for repeated hemotransfusion, and the possible use of anti-inflammatory agents in the management of VOC. The mainstay of treatment for children with SCD is HU; however, there is no long-term data on the effect of HU on pulmonary function measurement. A key recommendation is a critical need for large-scale multi-center and longitudinal studies on pulmonary function in children with SCD living in Africa to understand better early determinants and effective preventive strategies for impaired lung function.
Ethical approval
This study was approved by the Human Research Ethics Committee of the University of Cape Town with approval number HREC 345/2018 October.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Small Grants from the Severe Malaria Research Group , KNUST.
References
- Sickle cell disease in Africa: An urgent need for longitudinal cohort studies. Lancet Glob Health. 2019;7:e1310-1.
- [CrossRef] [PubMed] [Google Scholar]
- How many people have sickle cell disease in the UK? J Public Health. 2018;40:e291-5.
- [CrossRef] [PubMed] [Google Scholar]
- Global epidemiology of sickle haemoglobin in neonates: A contemporary geo-statistical model-based map and population estimates. Lancet. 2013;381:142-51.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of sickle cell disease in Cape Town. S Afr Med J. 2012;102:753-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886-95.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of mortality in sickle cell disease in adults in France and England. Hematol J. 2002;3:56-60.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality in sickle cell disease-life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639-44.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: Risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645-51.
- [CrossRef] [PubMed] [Google Scholar]
- Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855-65.
- [CrossRef] [PubMed] [Google Scholar]
- Heterogeneity of respiratory disease in children and young adults with sickle cell disease. Thorax. 2018;73:575-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern of lung function is not associated with prior or future morbidity in children with sickle cell anemia. Ann Am Thorac Soc. 2016;13:1314-23.
- [CrossRef] [PubMed] [Google Scholar]
- Sickle-cell disease in Malawian children is associated with restrictive spirometry: A cross-sectional survey. Int J Tuberc Lung Dis. 2013;17:1235-8.
- [CrossRef] [PubMed] [Google Scholar]
- Lung function in children with sickle cell disease from Central Africa. Thorax. 2019;74:604-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary function abnormalities in Nigerian children with sickle cell anaemia: Prevalence, pattern and predictive factors. Pediatr Respirol Crit Care Med. 2018;2:73.
- [CrossRef] [Google Scholar]
- ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1600016.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of spirometry vs impulse oscillometry: A comparative study in children with sickle cell disease. Pediatr Pulmonol. 2019;54:1422-30.
- [CrossRef] [PubMed] [Google Scholar]
- Technical standards for respiratory oscillometry. Eur Respir J. 2020;55:1900753.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical correlates of acute pulmonary events in children and adolescents with sickle cell disease. Eur J Haematol. 2013;91:62-8.
- [CrossRef] [PubMed] [Google Scholar]
- Airway hyper reactivity is frequent in nonasthmatic children with sickle cell disease. Pediatr Pulmonol. 2016;51:950-7.
- [CrossRef] [PubMed] [Google Scholar]
- Airway hyper responsiveness does not predict morbidity in children with sickle cell anemia. Am J Respir Crit Care Med. 2017;195:1533-4.
- [CrossRef] [PubMed] [Google Scholar]
- Airway inflammation and lung function in sickle cell disease. Pediatr Allergy Immunol Pulmonol. 2019;32:92-102.
- [CrossRef] [PubMed] [Google Scholar]
- Phase three manual of the international study of asthma and allergies in childhood (ISAAC) Int J Tuberc Lung Dis. 2000;9:10-6.
- [Google Scholar]
- Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200:E70-88.
- [CrossRef] [PubMed] [Google Scholar]
- ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60:2101499.
- [CrossRef] [Google Scholar]
- Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur Respir Soc. 2012;40:1324-43.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxyurea therapy modulates sickle cell anemia red blood cell physiology: Impact on RBC deformability, oxidative stress, nitrite levels and nitric oxide synthase signalling pathway. Nitric Oxide. 2018;81:28-35.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemia-reperfusion injury in sickle cell disease: From basics to therapeutics. Am J Pathol. 2019;189:706-18.
- [CrossRef] [PubMed] [Google Scholar]
- Lung function in African infants in the drakenstein child health study. Impact of lower respiratory tract illness. Am J Respir Crit Care Med. 2017;195:212-20.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary function, CT and echocardiographic abnormalities in sickle cell disease. Thorax. 2014;69:746-51.
- [CrossRef] [PubMed] [Google Scholar]
SUPPLEMENTARY TABLE
| Disease | Kappa statistic | Interpretation |
|---|---|---|
| Air space disease | 0.4737 | moderate |
| Reticular disease | 0.4667 | moderate |
| Nodular disease | 1.0000 | Almost perfect |
| Bronchiectasis | 0.7619 | Substantial |
| Pleural abnormality | 1.0000 | Almost perfect |
| Cardiomegaly | 0.5349 | Moderate |
| The overall presence of any abnormality | 0.4615 | Moderate |







