Translate this page into:
Post-COVID-19 pneumonia: Long-term radiographic and spirometric outcomes
*Corresponding author: Esthel Lee Presley Bemba, Department of Pneumology, Centre Hopsitalier Universitaire de Brazzaville, Brazzaville, Congo. bemba1@gmx.fr
-
Received: ,
Accepted: ,
How to cite this article: Bemba EL, Okemba Okombi FH, Moyikoua R, Bopaka RG, Koumeka PP, Ossale-Abacka KB, et al. Post-COVID-19 pneumonia: Long-term radiographic and spirometric outcomes. J Pan Afr Thorac Soc. 2024;5:127-34. doi: 10.25259/JPATS_18_2024
Abstract
Objectives:
This study aimed to evaluate the long-term radiographic and spirometric outcomes in patients who survived Coronavirus Disease 2019 (COVID-19) pneumonia in Brazzaville, with a focus on identifying the prevalence and contributing factors to persistent pulmonary sequelae.
Materials and Methods:
A cross-sectional study was conducted in multiple COVID-19 treatment centers in Brazzaville. A total of 52 patients, with a median age of 49.5 years, were assessed at least six months after recovery. Thoracic computed tomography (CT) scans were used to evaluate radiographic abnormalities, while spirometry assessed ventilatory function. Factors contributing to these abnormalities were analyzed, including age, comorbidities, and the severity of the acute illness.
Results:
Radiographic analysis revealed that 42.3% of patients had abnormal thoracic CT scans, with common findings including ground-glass opacities (52.2%), atelectasis (39.1%), and traction bronchiectasis (13%). Spirometric analysis revealed that 71.1% of patients had ventilatory disorders and predominantly restrictive patterns (51.4%). Advanced age, comorbidities (such as diabetes and hypertension), and the use of mechanical ventilation were significantly associated with radiographic abnormalities. Persistent respiratory symptoms, including chronic cough and dyspnea, were reported by 32.7% of patients, and 44.2% experienced impaired quality of life.
Conclusion:
These findings underscore the importance of long-term follow-up for COVID-19 pneumonia survivors, particularly those with persistent symptoms or comorbidities. Regular monitoring of respiratory function through spirometry and imaging is essential to managing long-term sequelae and improving patients’ quality of life.
Keywords
Coronavirus disease 2019
Pulmonary sequelae
Spirometry
Computed tomography scan
INTRODUCTION
The Coronavirus Disease 2019 (COVID-19) pandemic, caused by the SARS-CoV-2 virus, has had a devastating global impact, leading to numerous severe respiratory complications. Among these, COVID-19 pneumonia has emerged as one of the most frequent and severe, often requiring prolonged hospitalizations and intensive care.[1] While the majority of patients recover from the acute phase of infection, a growing number of studies highlight the emergence of long-term pulmonary sequelae. These sequelae, which can persist for several months after clinical recovery, have a significant impact on patients’ pulmonary function and quality of life.[2]
Thoracic imaging, through examinations such as chest radiography and computed tomography (CT), has played a crucial role in managing patients with COVID-19 by tracking the progression of lung lesions. The most frequently observed abnormalities include ground-glass opacities, consolidations, and, in severe cases, signs of pulmonary fibrosis.[3] Even after clinical resolution of the infection, these abnormalities can persist, particularly in patients who suffer from severe forms of COVID-19 pneumonia.[4] In parallel, lung function assessments, primarily performed through spirometry, frequently reveal persistent ventilatory disorders in post-COVID patients. The most common spirometric profiles are restrictive, indicating a reduction in lung capacity, often linked to phenomena of fibrosis or lung remodeling.[5] However, obstructive and mixed disorders may also be observed, especially in patients with a history of smoking or pre-existing respiratory comorbidities.[6]
Given these findings, it is essential to better understand the long-term pulmonary sequelae in patients recovered from COVID-19 pneumonia to improve their medical care and follow-up. This study aims to answer the following question: What are the clinical, radiographic, and functional factors associated with long-term pulmonary sequelae in patients recovered from COVID-19 pneumonia in Brazzaville?
The main hypothesis is that patients who have suffered from COVID-19 pneumonia present persistent long-term radiographic and functional abnormalities. In addition, these sequelae are likely influenced by factors such as sociodemographic characteristics, pre-existing comorbidities, and the severity of the acute illness. It is also hypothesized that patients who experienced severe forms of COVID-19 pneumonia will have more marked impairments in their respiratory function.
In this context, the general objective of this study is to analyze the radiographic and functional sequelae in patients recovered from COVID-19 pneumonia in Brazzaville. More specifically, it aims to (1) describe the sociodemographic, clinical, functional, and morphological characteristics of patients recovered from COVID-19 pneumonia in Brazzaville and (2) identify the factors associated with impaired respiratory function and radiographic abnormalities in these patients.
MATERIALS AND METHODS
Study design and period
This was a cross-sectional multicenter study with prospective data collection conducted from “March 1st” to “September 1st”, 2022, over a 6-month period.
Study setting
The study was conducted across various COVID-19 treatment centers in Brazzaville:
The University Hospital of Brazzaville in the pulmonology and infectious disease departments
Albert Leyono Municipal Clinic
The Sino-Congolese Hospital of Mfilou.
Patients
The general population consisted of patients with a history of COVID-19 pneumonia living in Brazzaville.
Inclusion criteria
Patients with a history of COVID-19 pneumonia. The patients were included at least 6 months after recovery from COVID-19. The timeframe was calculated from the polymerase chain reaction (PCR) negativity to ensure that the sequelae had sufficiently progressed to be accurately identified both through imaging and spirometry
Hospitalized in one of the treatment centers
Aged 18 years or older, regardless of gender
Having telephone contact and consenting to participate in the study.
Non-inclusion criteria
Patients who had suffered from COVID-19 <6 months before the study
Patients with respiratory diseases diagnosed before their COVID-19 infection or with active/recent respiratory conditions.
We performed an exhaustive recruitment of patients meeting the above selection criteria.
Measurement tools and operational definitions
Spirometric measurements
We used a Minispir® spirometer with a turbine flow sensor by MIR, compliant with American Thoracic Society/European Respiratory Society standards. The Global Lung Initiative recommendations were used to define as follows:
Normal spirometry when forced expiratory volume in 1 sec (FEV1) ≥ lower limit of normal (LLN) and forced vital capacity (FVC) ≥ LLN of predicted values.
Obstructive ventilatory disorder when FEV1/FVC ≤ LLN.
Restrictive pattern when FEV1/FVC > LLN and FVC < LLN of predicted values.
Mixed ventilatory disorder when FVC < LLN and FEV1/FVC < LLN.
Radiological assessment
The radiological assessment was based on thoracic CT scans, which allowed the identification of various sequelae.
Study conduct
A month before the study began, we conducted a pre-test to assess the understanding, relevance, plausibility, and homogeneity of the items in the data collection form. The pre-test, conducted with 10 patients, confirmed that the questionnaire (including its phrasing and questions) posed no significant issues.
After the pre-test, patients were contacted individually by telephone using the registers and medical records from the COVID-19 treatment centers. During the telephone interview, the study was briefly explained, and appointments were scheduled at the pulmonology department of the University Hospital of Brazzaville for further clarification. At these appointments, patients presented their latest COVID-19 PCR results.
All patients received an information sheet about the study and signed an informed consent form before inclusion. A quick health check was performed to verify the patient’s overall health status, vital signs, and anthropometric data, as well as to detect any contraindications to spirometry testing.
Afterward, we completed the standardized questionnaire based on literature data and conducted spirometry in the pulmonology department. Patients were then scheduled for a CT scan, which was interpreted by a radiologist.
Statistical analyses
The data were analyzed using Epi Info software version 7. The Chi-square test or Fisher’s exact test was used for comparing categorical variables when necessary. Mean values were presented with standard deviation as a measure of dispersion. In general, differences were considered significant when the P < 0.05. Significant variables in univariate analysis (P < 0.05) were included in the multivariate analysis using logistic regression. In addition, certain clinically relevant variables, such as age, human immunodeficiency virus (HIV) infection, diabetes, and the use of antivirals, were included in the multivariate model, even if they did not reach statistical significance in univariate analysis. This allows for the control of potential confounding factors and the examination of complex interactions between variables, thereby strengthening the robustness of the model and ensuring a more accurate estimation of the effect of each factor on spirometric abnormalities.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was submitted to the National Ethics Committee for Health Research (CERSSA) of the Republic of Congo, with a favorable opinion number 0012/MESRSIT/DGRST/CERSSA/-22.
RESULTS
General data
Of the 322 patients initially identified as potentially eligible for the study, a total of 210 patients were successfully contacted. Among them, 130 declined to participate in the study, while 80 agreed. However, of these, 28 patients were excluded for various reasons, such as medical contraindications or unmet eligibility criteria. In the end, 52 patients were included in the analysis [Figure 1].
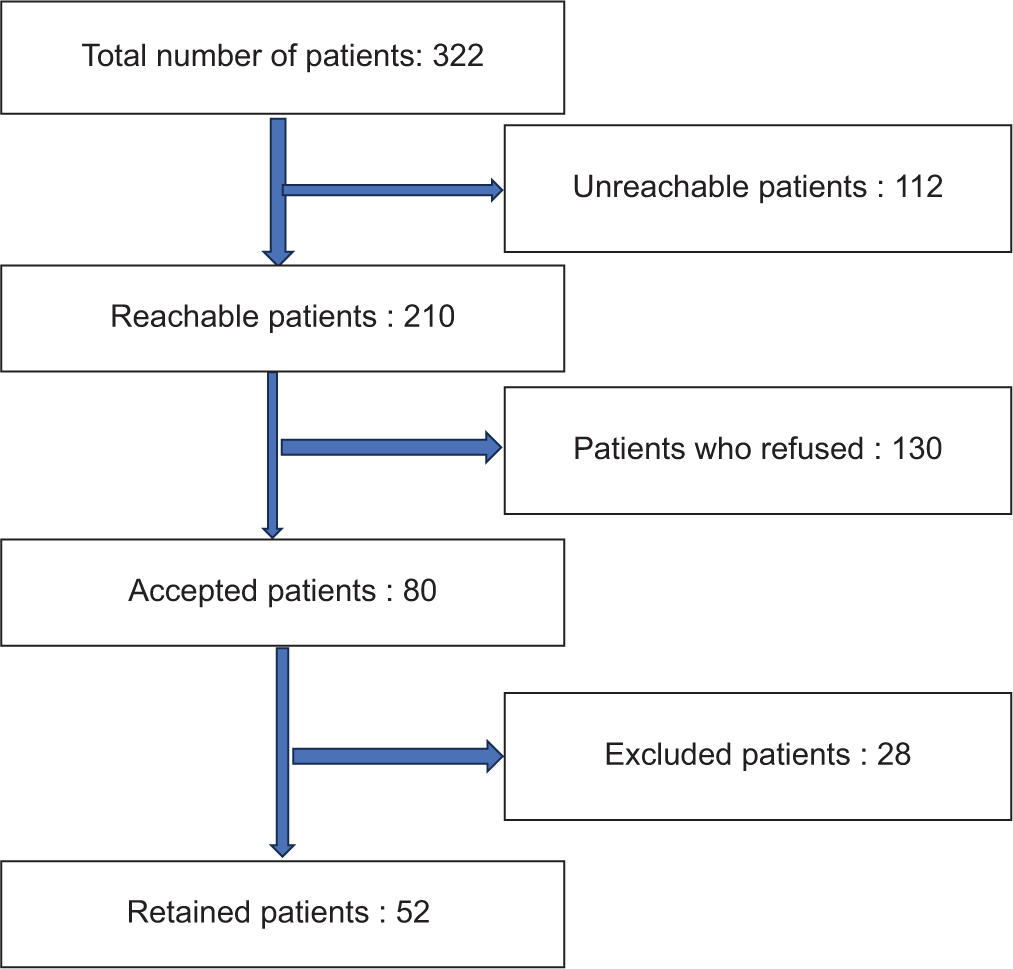
- Flow diagram.
The study included 52 patients, of whom 29 were men (56%) and 23 were women (44%), with a median age of 49.5 years (range: 21–86 years). The most represented age group was 45– 54 years, comprising 32.7% of the total sample. The average time since the infectious episode (pneumonia) was 8.2 ± 1.8 months. Hypertension was found in 40.4% of the cases, and diabetes mellitus in 19.2%. Eighteen (18) patients, or 34.6%, required mechanical ventilation during their pneumonia episode.
Out of the 52 patients, 17 (32.7%) presented persistent respiratory symptoms. Chronic cough and dyspnea were observed in 25% and 17.3% of cases, respectively. The mean St. George’s Respiratory Questionnaire score was 10.9. Patients with impaired quality of life numbered 23 (44.2%).
Functional findings
Of the 52 patients in our sample, 19 (36.5%) had normal spirometry results, while 37 (71.1%) exhibited ventilatory disorders. Restrictive patterns were the most prevalent, accounting for 51.4% (19/37) of the ventilatory disorders, as shown in Table 1. Tables 2 and 3 present the various factors associated with respiratory functional disorders.
| Ventilatory profile | Frequency | % of all ventilatory disorders | % of total population |
|---|---|---|---|
| Restrictive profile | 19 | 51.4% (19/37) | 36.5% (19/52) |
| OVD | 10 | 27% (10/37) | 19.2% (10/52) |
| Mild | 4 | 40% (4/10) | 7.7% (4/52) |
| Moderate | 4 | 40% (4/10) | 7.7% (4/52) |
| Severe | 2 | 20% (2/10) | 3.9% (2/52) |
| MVD | 8 | 21.6% (8/37) | 15.4% (8/52) |
| Parameters | Normal spirometry | Abnormal spirometry | OR [95% CI] | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 53.3% (8/15) | 40.5% (15/37) | 1 | |
| Female | 46.7% (7/15) | 59.5% (22/37) | 1.7 [0.5–5.6] | 0.3 |
| Age | ||||
| ≤60 years | 80% (12/15) | 73% (27/37) | 1 | |
| 60 years | 20% (3/15) | 27% (8/37) | 1.1 [0.2–4.9] | 0.6 |
| Diabetes mellitus | ||||
| Yes | 26.7% (4/15) | 18.9% (7/37) | 1 | |
| No | 73.3% (11/15) | 81.1% (30/37) | 1.6 [0.4–6.4] | 0.4 |
| Hypertension | ||||
| Yes | 40% (6/15) | 40.5% (15/37) | 1 | |
| No | 60% (9/15) | 59.5% (22/37) | 0.9 [0.3–3.3] | 0.6 |
| Cardiopathy | ||||
| Yes | 0% (0/15) | 8.1% (3/37) | 1 | 0.3 |
| No | 100% (15/15) | 91.9% (34/37) | ----------- | |
| HIV infection | ||||
| Yes | 33.3% (2/6) | 66.7% (4/6) | 1 | 0.78 |
| No | 50% (13/26) | 69.8% (32/46) | 2.2 [0.4–11.8] | |
| Remdesivir | ||||
| Yes | 40% (6/15) | 58.3% (7/12) | 1 | 0.21 |
| No | 60% (9/15) | 41.7% (5/12) | 1.5 [0.4–6.3] | |
| Lopinavir/Ritonavir | ||||
| Yes | 37.5% (3/8) | 62.5% (5/8) | 1 | 0.29 |
| No | 62.5% (5/8) | 37.5% (3/8) | 1.8 [0.5–7.2] | |
| Hydroxychloroquine/Chloroquine | ||||
| Yes | 44.4% (8/18) | 58.3% (7/12) | 1 | 0.34 |
| No | 55.6% (10/18) | 44.4% (8/18) | 1.3 [0.5–5.1] | |
| NIV | ||||
| Yes | 20% (3/15) | 40.5% (15/37) | 1 | 0.2 |
| No | 80% (12/15) | 59.5% (22/37) | 2.7 [0.6–12.2] |
NIV: Non-invasive ventilation, HIV: Human immunodeficiency virus, OR: Odds ratio, CI: Confidence interval
| Parameters | Normal spirometry | Abnormal spirometry | OR [95% CI] | P-value |
|---|---|---|---|---|
| Consultation Delay | ||||
| 6–7 months | 53.3% (8/15) | 37.8% (14/37) | 1 | |
| ≥7 months | 46.7% (7/15) | 62.2% (23/37) | 1.9 [0.5–6.3] | 0.23 |
| BMI (kg/m2) | ||||
| 18.5–24.9 | 46.7% (7/15) | 40.5% (15/37) | 1 | |
| ≥25 | 53.3% (8/15) | 59.5% (22/37) | 1.28 [0.4–4.3] | 0.5 |
| Cough | ||||
| Yes | 46.7% (7/15) | 18.9% (7/37) | 1 | |
| No | 53.3% (8/15) | 81.1% (30/37) | 3.7 [1.01–13.8] | 0.04 |
| Dyspnea | ||||
| Yes | 40% (6/15) | 10.8% (4/37) | 1 | |
| No | 60% (9/15) | 89.2% (33/37) | 5.5 [1.3–23.8] | 0.02 |
| Bronchorrhea | ||||
| Yes | 13.3% (2/15) | 2.7% (1/37) | 1 | |
| No | 86.7% (13/15) | 97.3% (36/37) | 1.28 [0.02–47.6] | 0.5 |
| Quality of life | ||||
| Good | 53.3% (8/15) | 35.1% (13/37) | 1 | |
| Impaired | 46.7% (7/15) | 64.9% (24/37) | 1.21 [0.6–7.1] | 0.2 |
| CT scan | ||||
| Normal | 46.7% (7/15) | 56.8% (21/37) | 1 | |
| Abnormal | 53.3% (8/15) | 43.2% (16/37) | 0.9 [0.3–3.0] | 0.5 |
BMI: Body mass index, CT: Computed tomography, OR: Odds ratio, CI: Confidence interval
Radiographic findings
Among the 52 thoracic CT scans performed, 22 (42.3%) were abnormal, while 30 (57.7%) were normal. The most frequent lesions observed were ground-glass opacities (52.2%), followed by atelectasis (39.1%) and traction bronchiectasis (13%). Figure 2 illustrates the different types of pulmonary lesions observed. Tables 4 and 5 report the factors related to radiographic results.
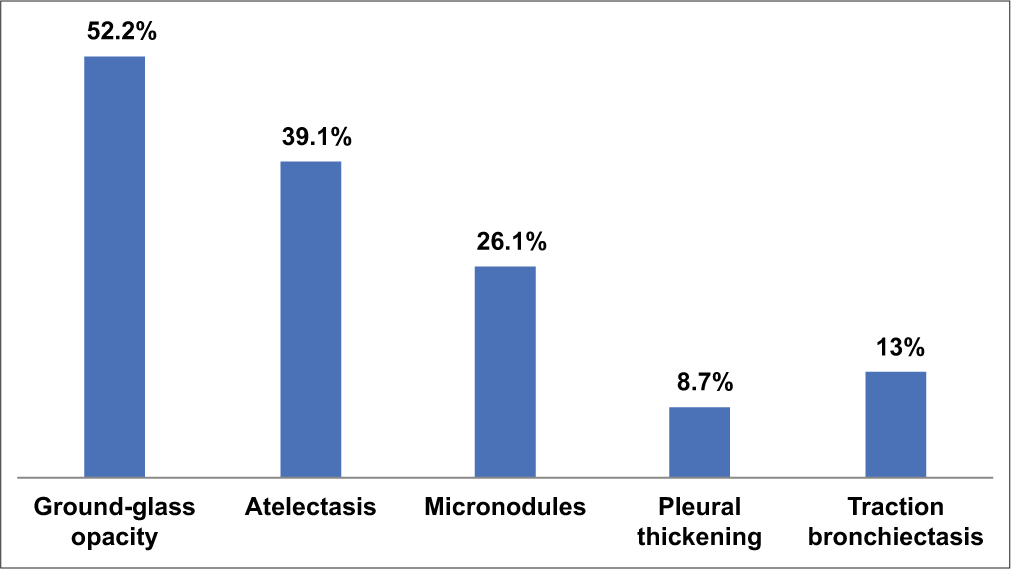
- Various pulmonary lesions on thoracic computed tomography scan.
| Parameters | Normal CT Scan | Abnormal CT Scan | OR [95% CI] | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 50% (8/16) | 48% (12/25) | 1 | 0.65 |
| Female | 50% (8/16) | 52% (13/25) | 1.1 [0.4–3.2] | |
| Age | ||||
| ≤60 years | 75% (12/16) | 68% (17/25) | 1 | 0.43 |
| >60 years | 25% (4/16) | 32% (8/25) | 1.4 [0.5–4.1] | |
| Diabetes mellitus | ||||
| Yes | 25% (4/16) | 40% (10/25) | 2.0 [0.6–7.0] | 0.2 |
| No | 75% (12/16) | 60% (15/25) | 1 | |
| Hypertension | ||||
| Yes | 38% (6/16) | 44% (11/25) | 1.4 [0.4–5.2] | 0.55 |
| No | 62% (10/16) | 56% (14/25) | 1 | |
| HIV infection | ||||
| Yes | 20% (3/15) | 38% (6/16) | 2.4 [0.5–11.8] | 0.3 |
| No | 80% (12/15) | 62% (10/16) | 1 | |
| Remdesivir use | ||||
| Yes | 40% (6/15) | 55% (7/15) | 1.8 [0.5–7.5] | 0.28 |
| No | 60% (9/15) | 45% (5/15) | 1 | |
| Lopinavir/Ritonavir use | ||||
| Yes | 37.5% (3/8) | 62.5% (5/8) | 2.2 [0.6–9.0] | 0.34 |
| No | 62.5% (5/8) | 37.5% (3/8) | 1 | |
| Hydroxychloroquine/Chloroquine use | ||||
| Yes | 45% (8/18) | 55% (10/18) | 1.3 [0.5–5.1] | 0.34 |
| No | 55% (10/18) | 45% (8/18) | 1 |
HIV: Human immunodeficiency virus, CT: Computed tomography, OR: Odds ratio, CI: Confidence interval
| Parameters | Normal CT scan | Abnormal CT scan | OR [95% CI] | P-value |
|---|---|---|---|---|
| Consultation delay | ||||
| 6–7 months | 53.3% (8/15) | 37.8% (14/37) | 1 | 0.23 |
| ≥7 months | 46.7% (7/15) | 62.2% (23/37) | 1.9 [0.5–6.3] | |
| BMI (kg/m2) | ||||
| 18.5–24.9 | 46.7% (7/15) | 40.5% (15/37) | 1 | 0.5 |
| ≥25 | 53.3% (8/15) | 59.5% (22/37) | 1.28 [0.4–4.3] | |
| Cough | ||||
| Yes | 46.7% (7/15) | 18.9% (7/37) | 1 | 0.04 |
| No | 53.3% (8/15) | 81.1% (30/37) | 3.7 [1.01–13.8] | |
| Dyspnea | ||||
| Yes | 40% (6/15) | 10.8% (4/37) | 1 | 0.02 |
| No | 60% (9/15) | 89.2% (33/37) | 5.5 [1.3–23.8] | |
| Bronchorrhea | ||||
| Yes | 13.3% (2/15) | 2.7% (1/37) | 1 | 0.5 |
| No | 86.7% (13/15) | 97.3% (36/37) | 1.28 [0.02–47.6] | |
| Quality of life | ||||
| Good | 53.3% (8/15) | 35.1% (13/37) | 1 | 0.2 |
| Impaired | 46.7% (7/15) | 64.9% (24/37) | 1.21 [0.6–7.1] | |
| Spirometry results | ||||
| Normal | 46.7% (7/15) | 56.8% (21/37) | 1 | 0.5 |
| Abnormal | 53.3% (8/15) | 43.2% (16/37) | 0.9 [0.3–3.0] |
BMI: Body mass index, CT: Computed tomography, OR: Odds ratio, CI: Confidence interval
Factors related to impairment of respiratory function and pulmonary sequelae [Tables 6 and 7] report the various factors.
| Factors | Adjusted OR [95% CI] | P-value |
|---|---|---|
| Age (> 60 years) | 1.7 [0.6–5.1] | 0.27 |
| Consultation delay (≥7 months) | 2.1 [0.8–6.0] | 0.10 |
| BMI (≥25 kg/m2) | 1.3 [0.4–5.0] | 0.50 |
| Presence of cough | 3.6 [1.0–12.8] | 0.04 |
| Presence of dyspnea | 4.4 [1.2–17.0] | 0.03 |
| Hypertension | 1.5 [0.4–6.0] | 0.40 |
| Diabetes mellitus | 2.1 [0.6–7.2] | 0.21 |
| HIV infection | 2.3 [0.5–9.5] | 0.29 |
| Use of remdesivir | 1.9 [0.5–7.1] | 0.30 |
| Use of lopinavir/ritonavir | 1.7 [0.5–6.0] | 0.32 |
| Use of hydroxychloroquine/chloroquine | 1.4 [0.5–5.0] | 0.35 |
| NIV | 2.8 [1.0–8.2] | 0.05 |
BMI: Body mass index, NIV: Non-invasive ventilation, HIV: Human immunodeficiency virus, CT: Computed tomography, OR: Odds ratio, CI: Confidence interval
| Factors | Adjusted OR | 95% CI | P-value |
|---|---|---|---|
| Age (> 60 years) | 1.8 | [1.1–3.2] | 0.04 |
| Comorbidities (Diabetes) | 2.1 | [0.9–4.6] | 0.07 |
| Comorbidities (Hypertension) | 1.5 | [0.8–3.2] | 0.2 |
| HIV Infection | 3.2 | [1.3–7.8] | 0.01 |
| Mechanical Ventilation (NIV) | 2.7 | [1.2–5.5] | 0.03 |
| Persistent Cough | 3.7 | [1.01–13.8] | 0.04 |
| Persistent Dyspnea | 5.5 | [1.3–23.8] | 0.02 |
| Use of Remdesivir | 1.4 | [0.6–3.1] | 0.15 |
| Use of Lopinavir/Ritonavir | 1.8 | [0.7–4.5] | 0.1 |
| Use of Hydroxychloroquine | 1.3 | [0.5–3.0] | 0.25 |
DISCUSSION
This study focuses on the long-term radiographic and spirometric sequelae in patients who survived COVID-19 pneumonia in Brazzaville. Our results show that a significant proportion of patients present with radiographic abnormalities and that more than 70% suffer from persistent ventilatory disorders, which is consistent with observations from other recent studies.
In our study, 42.3% of patients showed abnormalities on chest CT scans, the most common being ground-glass opacities (52.2%), atelectasis (39.1%), and traction bronchiectasis (13%). These results align with those of Han et al., who reported that ground-glass opacities are frequent radiological characteristics in patients who have recovered from COVID-19.[7] Pulmonary fibrosis, observed in 20% of patients, is a feared complication that leads to increased lung stiffness and chronic respiratory disorders, confirming the findings of Myall et al.[8]
Regarding respiratory function, 71.1% of patients had ventilatory disorders, with a predominance of restrictive disorders (51.4%). These results are consistent with those of Mo et al., who showed that restrictive ventilatory disorders are common in patients who survived severe COVID-19 pneumonia.[4] Obstructive disorders (19.2%), although less frequent, were mainly associated with cases of bronchiectasis or a history of smoking.[5]
Several risk factors were identified in our study. Advanced age (>60 years) and the presence of comorbidities such as diabetes, hypertension, and HIV infection were strongly associated with spirometric and radiographic abnormalities. Patients living with HIV, in particular, showed an increased risk of developing persistent pulmonary sequelae, a finding corroborated by the studies of Gupta et al., who highlighted the link between HIV-related immunosuppression and the severity of post-COVID complications.[9] These results underscore the importance of close monitoring for immunocompromised patients, who are more vulnerable to long-term pulmonary sequelae.
Our study revealed that 32.7% of patients continued to experience persistent respiratory symptoms, such as chronic cough and dyspnea, 6 months after their initial recovery. These persistent symptoms are indicators of prolonged functional sequelae. Persistent dyspnea was strongly associated with spirometric abnormalities (adjusted odds ratio 5.5), a result that aligns with the study by Huang et al., which shows that dyspnea and fatigue are frequent symptoms in post-COVID-19 patients.[10]
These persistent symptoms may be due to chronic pulmonary lesions, such as fibrosis and bronchiectasis, as well as unresolved ventilatory disorders. It is essential to monitor these patients to quickly detect and treat persistent symptoms and underlying abnormalities.
Pulmonary rehabilitation is a crucial intervention to improve the quality of life of post-COVID patients with persistent symptoms. Contrary to a common misconception, pulmonary rehabilitation does not directly treat fibrotic lesions, but it helps improve functional capacity and exercise tolerance. Spruit et al. showed that pulmonary rehabilitation helps improve functional outcomes, reduce respiratory symptoms, and enhance the quality of life of patients with post-infectious pulmonary sequelae.[11]
Although not all patients in our study underwent pulmonary rehabilitation programs, this approach should be encouraged, especially for patients with persistent symptoms such as dyspnea or reduced exercise tolerance.
Regarding the use of antivirals, patients treated with remdesivir or lopinavir/ritonavir did not show significant differences in terms of persistent pulmonary sequelae. This observation is consistent with the study by Beigel et al., which demonstrated that while remdesivir accelerates clinical recovery, its impact on long-term sequelae remains limited.[12]
The use of chloroquine and hydroxychloroquine did not show any significant effect on radiographic or spirometric outcomes in our study. These results are consistent with the conclusions of Cavalcanti et al., who found no significant benefit of these drugs in patients with moderate-to-severe COVID-19.[13]
The results of our study highlight the importance of long-term follow-up for patients who survived COVID-19 pneumonia, particularly those with comorbidities such as HIV or persistent symptoms such as dyspnea and cough. Regular use of spirometry and thoracic imaging is essential to monitor lung function and quickly detect sequelae. As recommended by George et al., close follow-up and early interventions, such as pulmonary rehabilitation, are necessary to minimize the long-term effects on the respiratory health of patients.[6]
This study has several limitations. The small sample size (52 patients) limits the statistical power and generalizability of the results. Moreover, the absence of a non-COVID control group prevents attributing all observed abnormalities exclusively to SARS-CoV-2 infection. Studies by Goërtz et al. also highlighted the variability in post-COVID cases, complicating definitive conclusions on long-term pulmonary sequelae.[5]
CONCLUSION
This study shows that patients who survived COVID-19 pneumonia in Brazzaville frequently present with radiographic sequelae (ground-glass opacities and pulmonary fibrosis) and restrictive ventilatory disorders. Advanced age, comorbidities (hypertension, diabetes, and HIV), and the use of mechanical ventilation are strongly associated with these abnormalities.
Severe forms of COVID-19 increase the risk of pulmonary sequelae, with persistent symptoms such as cough and dyspnea. Long-term follow-up, including spirometry, thoracic imaging, and pulmonary rehabilitation, is essential to improving the quality of life of vulnerable patients.
Ethical approval
The research/study was approved by the Institutional Review Board at Health Sciences Research Ethics Committee, number 12/MESRSIT/DGRST/CERSSA/-22, dated November 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- A novel coronavirus from patients with pneumonia in China 2019. N Engl J Med. 2020;382:727-33.
- [CrossRef] [PubMed] [Google Scholar]
- Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan China: A descriptive study. Lancet Infect Dis. 2020;20:425-34.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020;6:542-2020.
- [CrossRef] [PubMed] [Google Scholar]
- Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177-86.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent post-COVID-19 interstitial lung disease: An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18:799-806.
- [CrossRef] [PubMed] [Google Scholar]
- 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220-32.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society and American Thoracic Society-coordinated international task force. Eur Respir J. 2020;56:2002197.
- [CrossRef] [PubMed] [Google Scholar]
- Remdesivir for the treatment of Covid-19-final report. N Engl J Med. 2020;383:1813-26.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383:2041-52.
- [CrossRef] [PubMed] [Google Scholar]






