Translate this page into:
Oxidative stress and gene expression of antioxidants enzymes in monocrotaline-induced pulmonary hypertension following the administration of antiretroviral medications in rats
*Corresponding author: Adekunle Olatayo Adeoti, Department of Human Physiology, College of Health Sciences, University of KwaZulu Natal, Durban, South Africa. kadeoti2002@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Adeoti AO, Nadar A, Channa ML. Oxidative stress and gene expression of antioxidants enzymes in monocrotaline-induced pulmonary hypertension following the administration of antiretroviral medications in rats. J Pan Afr Thorac Soc 2022;3:85-91.
Abstract
Objectives:
Pulmonary hypertension (PH) is a severe life-threatening pulmonary vasculopathy. This study investigated the effects of zidovudine (AZT) and/or ritonavir (RTV) in the oxidative process of monocrotaline (MCT)-induced PH in rats.
Materials and Methods:
Forty male Sprague-Dawley rats weighing between 200 and 250 g were randomized into five different groups (n = 8 per group). A single dose intraperitoneal injection of MCT (60 mg/kg) was administered to all the rats in four of the groups. Daily oral administration of the antiretroviral (ARV) medications – (1) AZT-only (100 mg/kg); (2) RTV only (30 mg/kg); (3) AZT+ RTV (100+30 mg/kg); and (4) the untreated group had equivalent volume of saline for 28 days, respectively, while the (5) control group had neither MCT nor ARV. Gene expression using RT-PCR for the antioxidants and laboratory assay for lipid peroxidation was analyzed.
Results:
A significantly higher mRNA gene expression of catalase, superoxide dismutase and glutathione peroxidase in the treated rats was observed compared to the untreated. There was an increase in malondialdehyde (MDA) in the heart tissues of untreated rats (37.01 ± 1.16 nmol/g, P < 0.0001) compared to the control group (3.46 ± 0.97 nmol/g) with an associated reduction in MDA by the ARVs. Furthermore, an increase in the total antioxidant capacity in AZT (0.85 ± 0.02 nmol/g, P < 0.0001), RTV (0.63 ± 0.03 nmol/g, P < 0.0001), and combination of AZT/RTV (0.77 ± 0.06 nmol/g, P < 0.0001) compared to untreated (0.28 ± 0.03) rats.
Conclusion:
AZT and RTV ameliorate PH in experimental rats. This study demonstrated that MCT-induced PH generates ROS in rats and the protective role of ARV drugs in the treatment of PH.
Keywords
Monocrotaline
Oxidative stress
Pulmonary hypertension
Reactive oxygen species
INTRODUCTION
Pulmonary hypertension (PH) is a severe and life-threatening disease characterized by progressive vasculopathy and remodeling of pulmonary arteries leading to sustained pressure elevation in the pulmonary circulation.[1] Pulmonary artery remodeling is vital in the development of PH and right ventricular hypertrophy which involves oxidative reactions, mitochondrial injury, inflammation, and apoptosis.[2]
Oxidative stress is one of the identified factors, of the several complex processes, that culminate in the vascular remodeling toward the development and progression of PH.[3] An imbalance in the reactive oxygen species (ROS) handling due to either the overproduction of ROS and/or decreased capability of antioxidant defenses result in vasoconstriction through smooth muscle cell contraction due to a rise in cytosolic free Ca2+ concentration.[4] An upregulation of ROS in the pathogenesis of PH has been reported in both animal models and humans with PH.[5-7]
HIV-associated PH (HIV-PH) is a recognized complication of HIV/AIDS. The prevalence of HIV-PH has remained stable in the developed countries in developed countries before and after the advent of antiretroviral (ARV) drugs. However, the reported prevalence of HIV-PH is known to be relatively higher in sub-Saharan Africa countries.[8,9] The pathogenesis of HIV-PH remains unclear and there is no evidence that HIV infects pulmonary artery endothelial cells neither does its proteins accumulate insignificant amount in pulmonary vascular tissues during this process.[10]
Furthermore, there is ambiguity in the role of the ARV medications in the development and progression of the disease which has been reported in some studies to decrease pulmonary arterial pressure.[10,11] The demonstration of some ARVs with the different mechanisms of action, such as zidovudine (AZT) and indinavir in animal models, were reported to induce PH through endothelin-1 production, endothelial mitochondrial dysfunction, and excessive ROS generation.[12,13] Nonetheless, AZT also has potent anti-inflammatory and antioxidant activities while protease inhibitors (PIs) like ritonavir (RTV) have demonstrated a protective role in an animal model of HIV-PH[14,15] Likewise, several translational studies have shown the beneficial effect of ARVs medications in HIV-PH risk reduction and the variable outcomes among established early-stage HIV-PH patients.[16,17] This present study was conducted to identify the role of ARV medications especially AZT and RTV, in the oxidative process of monocrotaline (MCT)-induced PH in rats. Hence, the study investigated the hypothesis that regular administration of ARV medications in MCT-treated rats ameliorates PH through the oxidative stress-dependent mechanism.
MATERIALS AND METHODS
Animals
The animal experiment was approved by the Animal Research Committee of the University of KwaZulu-Natal, South Africa with the approval number AREC/066/018M. The study was conducted in accordance with the standard protocol and the ARRIVE guideline at the Biomedical Resource Centre at the University of KwaZulu-Natal, South Africa.[18] Forty adult male Sprague Dawley rats (200–250 g) were housed at 18–22°C under a 12-h light/12-h dark cycle with free access to food and water.
Experimental design
The 40 adult male Sprague-Dawley rats were randomly assigned into five groups. One of the groups (n = 8) served as the control while the remaining 32 rats were the PH model group that received a single 60 mg/kg intraperitoneal dose of MCT (Replamed, South Africa). Similarly, the rats in the control group were injected with the equivalent volume of sterile saline. The rats in the treatment group were randomized into four subgroups (n=8 per group). Following the administration of MCT, rats in the treatment groups were dosed once daily on the ARV drugs of AZT (100 mg/kg), RTV (30 mg/kg), or a combination of both AZT/RTV (100 mg/kg/30 mg/kg) or saline administration over a period of 4 weeks. The experiment was concluded on the 28th day and the rats were sacrificed by decapitation where the rats’ heart and lung tissues were harvested. There were five groups: (1) Control; (2) MCT + AZT; (3) MCT + RTV; (4) MCT + AZT + RTV, and (5) MCT alone.
Measurements of oxidative stress
The heart tissues were homogenized in ice-cold saline (0.9%) in a glass homogenizer; thereafter the homogenate was centrifuged at 2500 r/min for 10 min at 4°C temperature. The supernatant of homogenate (10%, w/v) was employed for the subsequent tests. Total antioxidant capacity (TAC) activity was determined using the colorimetric assay. TAC determination (ElabScience, Biotechnology, MD, USA) was carried out following the manufacturer’s protocol. While malondialdehyde (MDA) assay (Oxis Research, Portland, OR, USA) was demonstrated using the colorimetric quantification as a marker of lipid peroxidation. MDA concentration (µM) was measured at 532 nm and calculated using an MDA standard curve. The activities of MDA and TAC in the supernatant were determined according to the instructions of the respective testing kit (TAC assay kit, ElabScience, Biotechnology, MD, USA) and a microplate reader (Spectrostar Nano Bmg Labtech, Germany).
Morphometric analysis
The harvested rats’ lungs were immersed in 10% neutral buffered formalin and embedded in paraffin. Tissues were sectioned at 4 µm, stained with hematoxylin and eosin, and analyzed. A computerized morphometric system (Leica DM 500, Germany) was connected to light microscopy for the assessment of the slides. The slides were evaluated by light microscopy at ×400 magnification, and the reviewers (AO and AN) who assessed the extent of vascular remodeling were blinded to the grouping. We randomly selected ten fields containing terminal arterioles (50–150 mm external diameters) and the dimensions of the external elastic lamina and the internal elastic lamina of each vessel were measured. The wall thickness (percent) of the arterioles was calculated using the formula as wall thickness (%) = 100 × (1–internal elastic lamina/external elastic lamina).[19]
Quantitative real-time PCR
Total RNA was isolated from the rat heart using TRIzol (Zymo Research), subsequently transcribed into cDNA, and qPCR was performed. Primers for catalase (CAT) (5’-CCTCAGAAACCCGATGTCCTG-3’ and 5’- GTCAAAGTGTGCCATCTCGTCG -3’), MnSOD (5’-ACCGAGGAGAAGTACCACGA-3’ and 5’- TAGGGCTCAGGTTTTGTCCAG -3’), GPX-1 (5’-TGAGAAGTGCGAGGTGAATG-3’ and 5’- CGGGGACCAAATGATGTACT-3’) and GAPDH (5’-TGATTCTACCCACGGCAAGTT-3’, and 5’-TGATGGGTTTCCCATTGATGA-3’) were purchased from Inqaba (South Africa). Primers for gene expression analysis were designed using published sequence information (Ren et al., 2019).[20] PCR was performed in a 0.1 mL tube containing 2 µL cDNA, 1 µL (10 pmol) rat forward primer and reverse primer, 2 µL dNTP (1.25 mM each nucleotide), 5 µL SybrGreen, and 1µL dH2O. After denaturation at 95°C for 10 min, the mixture underwent PCR for 40 cycles at 95°C for 60 s, annealing temperature for 60 s, and elongation at 72°C for 60 s. The relative expression of protein mRNA level to GAPDH mRNA was calculated from the cycle threshold value by a ΔΔCq method and presented as relative to control.
Drugs
The MCT was purchased from Replamed (South Africa), while AZT from Aspen Pharmacare Limited (Aspen, South Africa), and RTV from Abbvie Limited (Abbvie, South Africa). The dosage of the medications was based on previous studies involving rats.[15,21] Of the major classes of ARV drugs, nucleoside reverse transcriptase inhibitors, which include AZT and PIs like RTV, have been the most widely implicated drug classes in the development of endothelial dysfunction.[22]
Statistical analysis
Statistical differences were analyzed by one-way ANOVA with Tukey multiple comparison post-test. Data were recorded as mean ± SEM with P < 0.05 considered significant. All analyses were performed using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA).
RESULTS
Assessment of lipid peroxidation
[Figure 1] shows a significant increase in the level of MDA in the heart tissues of experimental rats treated with MCT (37.01 ± 1.16 nmol/g, P <0.0001) compared to the rats in the control group (3.46 ± 0.97 nmol/g). The administration of AZT (8.80 ± 1.21 nmol/g, P < 0.0001), RTV (8.47 ± 1.24 nmol/g, P < 0.0001), and a combination of both drugs (9.18 ± 0.98 nmol/g, P < 0.0001) was associated with mitigation of the effect of MCT by the reduction in the MDA levels in the heart of treated rats.

- Level of lipid peroxidation in rat heart tissue. Values are expressed as mean ± SEM (n = 8/group). In comparison with the control: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 while in comparison with MCT #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001. MCT + DRUG COMBO represents MCT plus combination of Zidovudine and Ritonavir.
Assessment of TAC
There was a significant difference in the TAC of the heart tissue of the rats in the control group compared to the treatment group exposed to only MCT, without any ARV drugs given as shown in [Figure 2]. A significant increase in TAC levels in the heart tissues of the rats treated with AZT (0.85 ± 0.02 nmol/g, P < 0.0001), RTV (0.63 ± 0.03 nmol/g, P < 0.0001), or a combination of both drugs (0.77 ± 0.06 nmol/g, P < 0.0001) was observed when compared to the group administered only MCT (0.28 ± 0.03).
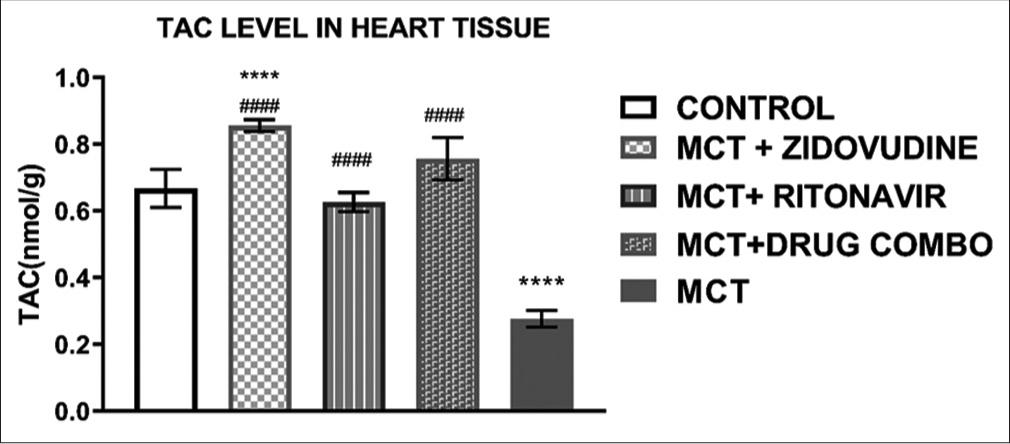
- Total antioxidant capacity of the rat heart tissues. Values are expressed as mean ± SEM (n = 8/group). In comparison with the control: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 while in comparison with MCT #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001. MCT + DRUG COMBO represents MCT plus combination of Zidovudine and Ritonavir.
Relative mRNA expression of superoxide dismutase (SOD) in the rat heart tissue
[Figure 3] shows the relative mRNA expression of SOD in the heart tissue of rats exposed to only MCT was significantly lower (1.39 ± 0.14, P < 0.0001) than in the control group (6.02 ± 0.17). MCT exposed animals treated with AZT (6.25 ± 0.26, P < 0.0001), RTV (5.78 ± 0.25, P < 0.0001), and a combination of both drugs (6.45 ± 0.42, P < 0.0001) expressed a significantly higher level of SOD mRNA in comparison to the animals exposed to MCT without any treatment with ARV drugs.
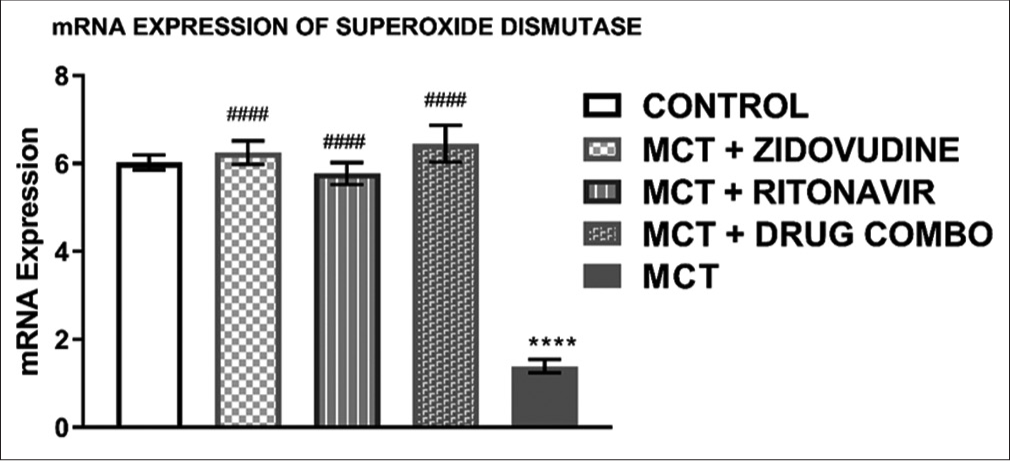
- Relative mRNA expression of superoxide dismutase in the rat heart tissue. Values are expressed as mean ± SEM (n = 8/group). In comparison with the control: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 while in comparison with MCT #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001. MCT + DRUG COMBO represents MCT plus combination of Zidovudine and Ritonavir.
Relative mRNA expression of CAT in the rat heart tissue
The relative mRNA expression of CAT in the heart tissue of animals exposed to only MCT was significantly lower (0.91 ± 0.06, P < 0.0001) than in the control group (1.88 ± 0.15, P < 0.0001). It was also observed that MCT exposed animals treated with AZT (1.99 ± 0.09, P < 0.0001), RTV (1.90 ± 0.02, P < 0.0001), and a combination of both drugs (1.96 ± 0.04, P = 0.0001) expressed a significantly higher level of CAT mRNA in comparison to the animals exposed to MCT without treatment with any ARV drugs as shown in [Figure 4].
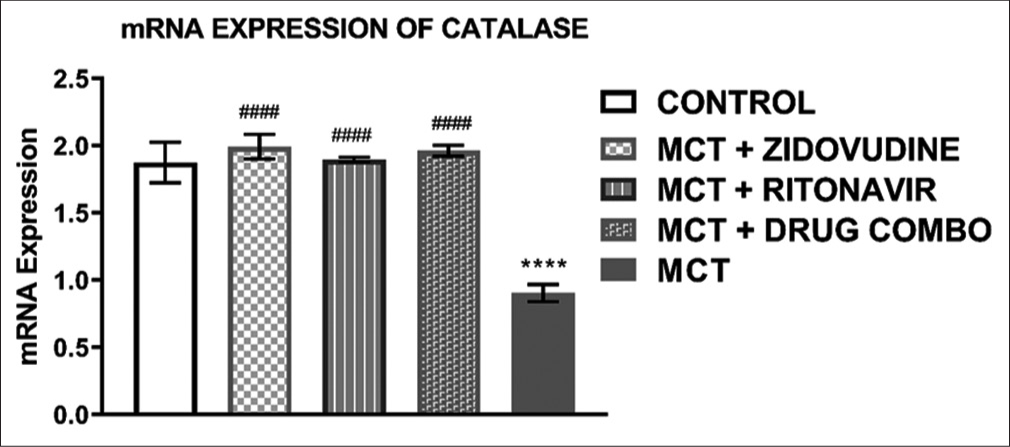
- Relative mRNA expression of catalaze in the heart tissue of experimental rats. Values are expressed as mean ± SEM (n = 8/group). In comparison with the control: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 while in comparison with MCT #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001. MCT + DRUG COMBO represents MCT plus combination of Zidovudine and Ritonavir.
Relative mRNA expression of glutathione peroxidase in the rat heart tissue
The relative mRNA expression of glutathione peroxidase in the heart of experimental animals exposed to MCT only (0.51 ± 0.17, P < 0.0001) was significantly lower than that of the control group (2.255 ± 0.16) as shown in [Figure 5]. The level of glutathione peroxidase mRNA expressed in MCT animals treated with AZT (2.35 ± 0.19, P < 0.0001), RTV (2.06 ± 0.09, P < 0.0001), and a combination of both drugs (2.28 ± 0.19, P < 0.0001) was similar to that of the control group but it was significantly higher than the glutathione mRNA expressed in the heart of animals exposed to only MCT.
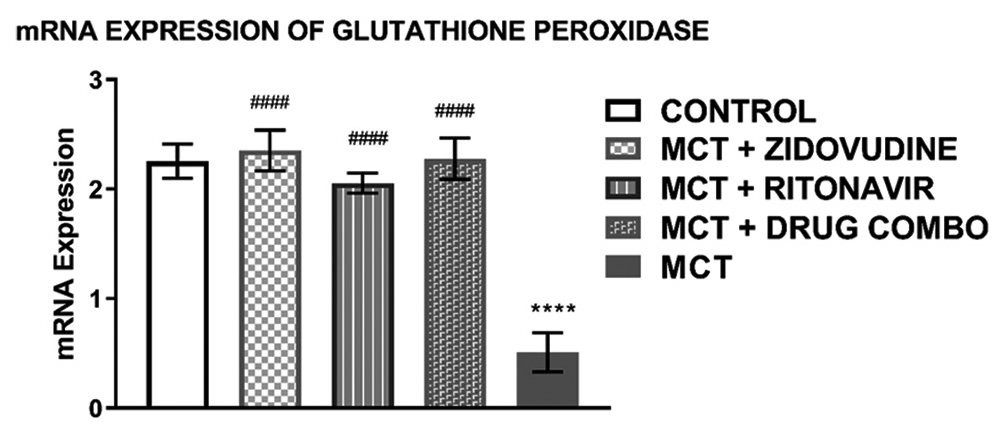
- mRNA expression of glutathione peroxidase in the heart tissue of experimental rats Values are expressed as mean ± SEM (n = 8/group). In comparison with the control: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 while in comparison with MCT #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001. MCT + DRUG COMBO represents MCT plus combination of Zidovudine and Ritonavir.
Animal survival rate
All the 40 rats survived the 1, 2, and 3 weeks and the rats in the control and treated groups remained active even till the end of the study. In contrast, three rats in the untreated group died in the 4th week of the study on days 23 and 25 with 38% death rate at the end of the experiment (P = 0.0097). During the study, none of the rats in the control group (unexposed) died as well as those on ARV medication, namely, an AZT, RTV, or a combination of both drugs.
Histological examination of the lung tissues of rats
Histological examination of the slides under the light microscope following H and E staining shows profound thickening in the media wall of the muscular pulmonary arteries in the lungs of rats treated with MCT. The arteries of rats treated with ARV medications were thicker than controls but not like those with only MCT as shown in [Figure 6].

- Representative images of lung stained with H and E for the different groups (Image a – Control, Image b – MCT+Zidovudine, Image c – MCT+Ritonavir, Image d – MCT+Drugs combination of Zidovudine and Ritonavir, and Image e – MCT).
The degree of arteriolar wall thickness in the different groups is demonstrated in [Figure 7]. The treatment group with MCT shows significantly higher percentage wall thickness than the control (67.47 ± 1.22% vs. 31.83 ± 1.09%, P < 0.0001). The ARV drug-AZT (41.17 ± 0.52% vs. 67.47 ± 1.22%, P < 0.0001), drug-RTV (39.83 ± 0.32% vs. 67.47 ± 1.22%, P < 0.0001), and a combination of both (AZT and RTV) drugs (39.53 ± 0.84% vs. 67.47 ± 1.22, P < 0.0001 when compared to MCT group significantly shows reduction in the percentage wall thickness.
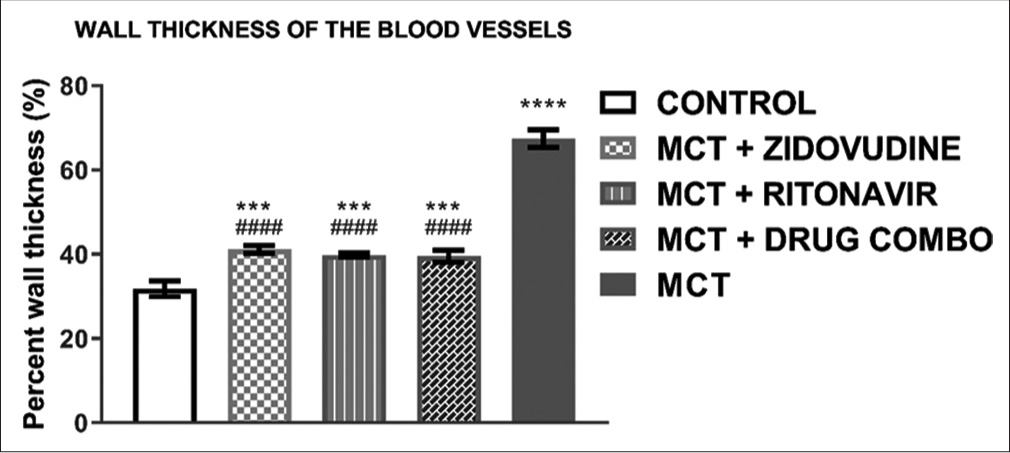
- Wall thickness of pulmonary arterioles in lung tissues of experimental rats. Values are expressed as mean ± SEM (n = 8/ group). In comparison with the control: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 while in comparison with MCT #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001. MCT + DRUG COMBO represents MCT plus combination of Zidovudine and Ritonavir.
DISCUSSION
This study showed the effects of ARVs on MCT-induced PH as it relates to oxidative stress. There was a decrease in MDA as evidence of lipid peroxidation and accompanying elevation in oxidative markers in the heart tissues of rats treated with ARV. This reduction in MDA levels while there were increased levels in TAC, SOD, CAT, and GPx was observed as well as the relative minimal thickness in the pulmonary arterioles of the lungs of MCT rats following the ARVs administration.
In our study, the critical period following the 4 weeks of intraperitoneal administration of MCT as observed in other studies showed the occurrence of death in rats, especially the untreated ones. This finding was similar to reports in the literature, for the establishment of the PH model in rats where after 28 days of the single dose of 60 mg/kg of MCT, there was an increased likelihood of mortality in rats.[23] This finding was due to the severity of the PH as it progresses beyond the 1st month of the experimental administration of MCT in the rats.
The role of ROS in the development and progression of PH following MCT administration has been well documented. Similarly, the mitigating effects of molecular hydrogen and anti-ROS medications have been demonstrated to improve MCT-induced PH in rats; however, the effects of ARV medication in this regard have not been explored.[6,23] The generation of ROS in MCT-injected rats is responsible for the development of PH which results from the reduction in nitric oxide (NO) levels and the proliferation of pulmonary arterial smooth muscle cells, which ultimately enhances pulmonary vasoconstriction and remodeling.[24,25]
In the study, marked thickening in the pulmonary arteriolar media walls was observed in the histopathology results of the lungs in the MCT treated rats compared to those treated with ARV medications. This observed finding was similar to the report in a previous study by Lee et al.[26] where prominent medial hypertrophy of the muscular pulmonary arteries was seen in MCT-induced PH rats. This structural change in the MCT treated rats when compared to the control and the rats that were administrated AZT and RTV could be explained by the mitigating effects of the ARV medications on MCT treated rats.
In animal models, increased MDA level has been reported in PH compared to controls and the degree of elevation of MDA has been correlated with the disease severity.[2,27,28] This similar finding was observed in our study, as the heart tissues of rats exposed to MCT showed elevated levels of MDA, which was significantly mitigated by administration of AZT, RTV, or both drugs in combination. This was likewise elucidated in the study by Feng et al., where an increased MDA level in MCT-induced PH in rats revealed that the administration of a redox regulatory agent was protective against PH.[24]
The overall balance between oxidants and antioxidants is reflected by the level of TAC which is an indicator of available enzymatic and non-enzymatic antioxidants in a tissue.[28] Our study found depletion in TAC in the MCT treated rats without ARV therapy while higher TAC in the groups on the ARV drugs.
As part of the main enzymatic antioxidant system, SODs catalyze the conversion of superoxide radicals to H2O2 while CAT further converts H2O2 to water. This activity can reverse pulmonary vascular remodeling and PH. Similar to our findings, significantly lower SOD mRNA expression has been reported in human and animal models.[29,30] In animal models, MCT induces oxidative stress by downregulating the expression of genes responsible for mopping up free radicals; hence, SOD preserves NO in vivo by scavenging superoxide and preventing the consumptive reactions.[29] In our study, the elevated SOD in the ARV-treated rats could have resulted in the rise in NO which prevented pulmonary vasoconstriction and ultimately averted the development of PH.
Similar findings were observed in the mRNA expression of CAT and GPX mRNA expression where MCT induced severe reduction in the GPX and CAT mRNA gene expression while these effects were significantly mitigated by the administration of the ARV medications, namely, AZT and RTV. An imbalance in the oxidative-antioxidative process in the blood vessel walls with increased ROS results in vasoconstriction, smooth muscle cell proliferation, and vascular remodeling, which if limited, could help alleviate PH.[31]
In this study, the administration of the ARV medications, namely, AZT, RTV, or the combination of the two drugs ameliorated the distortion in the oxidant-antioxidant system in rats exposed to MCT. However, this may not be sufficient as therapies for HIV-PH; hence, its combination with PH-specific therapy could be an adjuvant therapy with remarkable improvement. Furthermore, studies are also required to ascertain the role of oxidative stress following ARV medications in the reversal of established PH. In addition, this finding would encourage translational research in this critical area, especially in countries with a high burden of HIV/AIDS to identify the benefits of ARVs in the management of PH in this group of patients.
There is a limitation to this study that warrants discussion. The non-measurement of the right ventricular pressure by right heart catheterization, which is an invasive procedure, to establish an increased pressure in the right ventricle due to PH. However, the findings on histologic examination of the significant thickening of the media wall of pulmonary arteries in the MCT exposed rats but minimal thickening in the ARV treated rats gave an insight in this regard.
CONCLUSION
This study showed the generation of ROS in rats following MCT-induced PH. Also zidovudine and ritonavir ameliorated PH in the experimental rats. However, further research would be required to demonstrate such findings and the protective roles of ARVs in the development of PH in human subjects.
Acknowledgment
This study was supported by the research grant provided by the College of Health Sciences (CHS) of the University of KwaZulu-Natal, South Africa.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
This study was supported by the research grant provided by College of Health Sciences (CHS) of the University of KwaZulu-Natal, South Africa.
Conflicts of interest
There are no conflicts of interest.
References
- Inflammatory mediators drive adverse right ventricular remodeling and dysfunction and serve as potential biomarkers. Front Physiol. 2018;9:609.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium sulfate mitigates the progression of monocrotaline pulmonary hypertension in rats. Int J Mol Sci. 2019;20:4622.
- [CrossRef] [PubMed] [Google Scholar]
- Role of oxidized lipids in pulmonary arterial hypertension. Pulm Circ Sep. 2016;6:261-73.
- [CrossRef] [PubMed] [Google Scholar]
- Reactive oxygen species in pulmonary vascular remodeling. Compr Physiol. 2013;3:1011-34.
- [CrossRef] [PubMed] [Google Scholar]
- Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668-75.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress is associated with increased pulmonary artery systolic pressure in humans. Hypertension. 2014;63:1270-5.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1038-47.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of HIV-associated pulmonary hypertension: Potential role of HIV-1 Nef. Proc Am Thorac Soc. 2011;8:308-12.
- [CrossRef] [PubMed] [Google Scholar]
- HIV related pulmonary arterial hypertension: Epidemiology in Africa, physiopathology, and role of antiretroviral treatment. AIDS Res Ther. 2015;12:36.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary arterial hypertension related to HIV infection: Improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38:1178-85.
- [CrossRef] [PubMed] [Google Scholar]
- HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV cohort study. Am J Respir Crit Care Med. 1997;155:990-5.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of HIV drug combinations on endothelin-1 and vascular cell proliferation. Cardiovasc Toxicol. 2004;4:117-31.
- [CrossRef] [PubMed] [Google Scholar]
- HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicol Appl Pharmacol. 2007;224:60-71.
- [CrossRef] [PubMed] [Google Scholar]
- Zidovudine protects hyperosmolarity-stressed human corneal epithelial cells via antioxidant pathway. Biochem Biophys Res Commun. 2018;499:177-81.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of HIV protease inhibitors on progression of monocrotaline-and hypoxia-induced pulmonary hypertension in rats. Circulation. 2010;122:1937-47.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and risk factors associated with pulmonary hypertension in HIV-infected patients on regular follow-up. AIDS. 2012;26:1387-92.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of antiretroviral therapy on pulmonary hypertension in HIV patients. J Indian Med Assoc. 2013;111:845-6, 849
- [Google Scholar]
- Ensuring reproducibility and ethics in animal experiments reporting in Korea using the ARRIVE guideline. Lab Anim Res. 2018;34:11-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxysafflor yellow A improves established monocrotaline-induced pulmonary arterial hypertension in rats. J Int Med Res. 2016;44:569-84.
- [CrossRef] [PubMed] [Google Scholar]
- Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med Sci Monit. 2019;25:991-1000.
- [CrossRef] [PubMed] [Google Scholar]
- Naringin reverses hepatocyte apoptosis and oxidative stress associated with HIV-1 nucleotide reverse transcriptase inhibitors-induced metabolic complications. Nutrients. 2015;7:10352-68.
- [CrossRef] [PubMed] [Google Scholar]
- Azidothymidine (AZT) leads to arterial stiffening and intima-media thickening in mice. J Biomech. 2013;46:1540-7.
- [CrossRef] [PubMed] [Google Scholar]
- Hydrogen ameliorates pulmonary hypertension in rats by anti-inflammatory and antioxidant effects. J Thorac Cardiovasc Surg. 2015;150:645-54.e3.
- [CrossRef] [PubMed] [Google Scholar]
- Alginate oligosaccharide alleviates monocrotaline-induced pulmonary hypertension via anti-oxidant and anti-inflammation pathways in rats. Int Heart J. 2020;61:160-8.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative profiling of the failing right heart in rats with pulmonary hypertension. PLoS One. 2017;12:e0176887.
- [CrossRef] [PubMed] [Google Scholar]
- Monocrotaline-induced pulmonary hypertension correlates with upregulation of connective tissue growth factor expression in the lung. Exp Mol Med. 2005;37:27-35.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in patients with primary pulmonary hypertension. Bull Exp Biol Med. 2002;133:580-2.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of oxidative stress markers in patients with pulmonary arterial or chronic thromboembolic pulmonary hypertension. Oxid Med Cell Longev. 2019;2019:3795320.
- [CrossRef] [PubMed] [Google Scholar]
- Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clin Transl Sci. 2008;1:99-106.
- [CrossRef] [PubMed] [Google Scholar]
- PGC-1alpha induction in pulmonary arterial hypertension. Oxid Med Cell Longev. 2012;2012:236572.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of 18beta-glycyrrhetinic acid on monocrotaline-induced pulmonary arterial hypertension in rats. Front Pharmacol. 2019;10:13.
- [CrossRef] [PubMed] [Google Scholar]






