Translate this page into:
Global initiative for chronic obstructive lung disease 2023 report: Gold executive summary
*Corresponding author: Dr. Alvar Agustí, Institut Respiratori, Clinic Barcelona. C/Villarroel 170, 08036 Barcelona, Spain. Tel: +34 93 227 1701 E-mail: aagusti@clinic.cat
How to cite this article: Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: Gold executive summary. J Pan Afr Thorac Soc. 2024;5:92-114. doi: 10.2529/JPATS_GES_2023
INTRODUCTION
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has published the complete 2023 GOLD report, which can be freely downloaded from its web page (www.goldcopd.org) together with a “pocket guide” and “teaching slide set”[1]. It contains important changes compared to earlier versions, and incorporates 387 new references[1]. Here, we present an executive summary of this GOLD 2023 report[1] that summarizes aspects that a) are relevant from a clinician´s perspective and b) updates evidence published since the prior executive summary in 2017.
NEW DEFINITION OF COPD
The definition of a disease should only include the characteristics that distinguishes it from other diseases[2]. Accordingly, GOLD 2023 proposes a new definition of COPD that, at variance with previous documents[3], focuses exclusively on these characteristics, separately from its epidemiology, causes, risk factors and diagnostic criteria that are discussed on their own.
GOLD 2023 defines COPD as a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration and/or exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction.
PATHOGENESIS: CAUSES AND RISK FACTORS
COPD results from dynamic, cumulative and repeated gene (G) - environment (E) interactions over the lifetime (T) that damage the lungs and/or alter their normal development/aging processes (GETomics)[4].
Environmental risk factors
Cigarette smoking is a key environmental risk factor for COPD. Cigarette smokers have a higher prevalence of respiratory symptoms and lung function abnormalities, a greater annual rate FEV1 decline and a greater COPD mortality rate than non-smokers[5]; yet fewer than 50% of heavy smokers develop COPD[6]. Passive exposure to cigarette smoke, other types of tobacco smoke (e.g., pipe, cigar, water pipe)[7-9], and marijuana[10] are also risk factors for COPD[11]. Smoking during pregnancy poses a risk for the fetus, by altering lung growth and development in utero, and possibly priming the immune system for abnormal/enhanced responses in the future[4,12].
In low- and middle-income countries (LMICs), COPD in nonsmokers may be responsible for up to 60-70% of cases[13]. Because the LMICs contribute to over 85% of all COPD cases, non-smoking risk factors account for over 50% of the global burden of COPD[13]. Wood, animal dung, crop residues, and coal (i.e., biomass), typically burned in poorly functioning stoves, may lead to very high levels of household air pollution[14], which is associated with increased COPD risk, although the extent to which household air pollution versus other poverty-related exposures explain the association is unclear[15,16]. Compared to COPD in smokers, COPD nonsmokers is more common in females, of younger age, and exhibit similar (or milder) respiratory symptoms and quality of life impairment. They have similar spirometric indices but greater small airways obstruction, less emphysema and lesser rate of lung function decline. Finally they show lower neutrophil count, higher eosinophil numbers in the sputum[13] and a similar defect in macrophage phagocytosis of pathogenic bacteria[17]. Research is needed to understand how interventions aimed at decreasing household air pollution can reduce the risk of COPD as well as what is the most appropriate pharmacotherapy for this type of COPD[13].
Occupational exposures, including organic and inorganic dusts, chemical agents, and fumes, are an under-appreciated environmental risk factor for COPD[18,19]. The U.S. National Health and Nutrition Examination Survey III estimated the fraction of COPD attributable to workplace exposures was 19.2% overall, and 31.1% among never-smokers[20].
Air pollution, which typically consists of particulate matter (PM), ozone, oxides of nitrogen or sulfur, heavy metals, and other greenhouse gases, is a major worldwide cause of COPD, responsible for ~50% of the attributable risk for COPD in LMICs[1]. The risk of air pollution to individuals is dose-dependent with no apparent “safe” threshold. Even in countries with relatively low ambient air pollution levels, chronic exposure to PM<2.5 microns and nitrogen dioxides significantly impairs lung growth in children[21], accelerates lung function decline in adults and increases the risk for COPD, especially among those with additional risk factors[22]. Air pollution also increases the risk of COPD exacerbations, hospitalizations, and mortality[23].
Genetic risk factors
The most relevant genetic risk factor for COPD identified are mutations in SERPINA1, leading to α-1 antitrypsin deficiency, a major circulating inhibitor of serine proteases[24]. The PiZZ genotype affects 0.12% of COPD patients, and its prevalence ranges from 1/408 in Northern Europe to 1/1,274 in Eastern Europe[25]. There is no increased COPD risk in heterozygotes (MZ and SZ) in the absence of smoking[26].
Other genetic variants have also been associated with reduced lung function and risk of COPD, their individual effect size is small although their co-occurrence may increase disease susceptibility[27].
Lung function trajectories: Lung development and ageing
At birth, the lungs are not fully developed. They grow and mature until about 20-25 years of age (earlier in females), when lung function reaches its peak[5]. This is followed by a relatively short plateau and a final phase of mild lung function decline due to physiological lung aging. This normal lung function trajectory can be altered by processes occurring during gestation, birth, childhood, and adolescence that affect lung growth (hence, peak lung function) and/or processes shortening the plateau phase and/or accelerating the aging phase[28]. Indeed, in the general population there is a range of lung function trajectories through the lifetime[28]. Trajectories below the normal range are associated with a higher prevalence and earlier incidence of multi-morbidity and premature death[29], whereas those above the normal range are associated with healthier aging, fewer cardiovascular and respiratory events, as well as with a survival benefit[30,31].
Factors in early life termed “childhood disadvantage factors”, including prematurity, low birth weight, maternal smoking during pregnancy, repeated respiratory infections and poor nutrition, among others, are key determinants of peak lung function attained in early adulthood[32-39]. Reduced peak lung function in early adulthood increases the risk of COPD later in life[32,40,41]. In fact, approximately 50% of patients develop COPD due to accelerated decline in FEV1 over time while the other 50% develop it due to abnormal lung growth and development (with normal lung function decline over time)[42].
Sex
The prevalence of COPD in developed countries is now almost equal in males and females[43]. Women report more dyspnea, worse health status scores and have a higher incidence of exacerbations compared with men at similar severity of airflow limitation[44].
Socioeconomic status
Poverty and lower socioeconomic status are consistently associated with airflow obstruction[45] and increased risk of COPD[46]. It is likely that this reflects exposures to household and outdoor air pollutants, crowding, poor nutrition, infections, or other factors related to low socioeconomic status.
Asthma
Many different studies have reported that asthma and atopy in infancy may be a significant risk factor for COPD in adulthood[47,48]. However, it is important to remember that abnormal lung development in childhood and adolescence can cause asthma-like symptoms. Given that poor lung development is associated with COPD in adulthood (see above), some of these infants and adolescents may have been mislabeled as “asthma”.
Infections
Severe respiratory infections in childhood have been associated with reduced lung function and increased respiratory symptoms in adulthood[47,49]. In adults, chronic bronchial infection, particularly with Pseudomonas aeruginosa, is associated with accelerated FEV1 decline[50]. In many parts of the world, tuberculosis[51] and HIV infection[52] are also important risk factors for COPD.
DIAGNOSIS: FORCED SPIROMETRY
A diagnosis of COPD should be considered in any patient who complains of dyspnea, chronic cough or sputum production, a history of recurrent lower respiratory tract infections and/or a history of exposure to risk factors for the disease (see above) but forced vital capacity maneuver during spirometry showing the presence of a post-bronchodilator FEV1/FVC < 0.7 is needed to establish the diagnosis of COPD. The FEV1 also serves to determine the severity of airflow obstruction (GOLD grades 1,2,3, 4 or mild, moderate, severe, and very severe). Yet, several important aspects related to forced spirometry need to be considered here.
First, airflow obstruction that is not fully reversible is not specific for COPD. For instance, it may also be found in patients with asthma and other diseases, so the clinical context and risk factors (see above) must also be considered when establishing a diagnosis of COPD.
Second, if the post-bronchodilator FEV1/FVC ratio is between 0.60 and 0.80 on a single spirometric measurement, this should be confirmed by repeat spirometry on a separate occasion, as in some cases the ratio may change as a result of biological variation when measured at a later interval[53,54]. When the initial post-bronchodilator FEV1/FVC ratio is <0.6, it is very unlikely to rise spontaneously above 0.7.[53]
Third, there is a long-standing debate on whether it is better to use a fixed FEV1/FVC ratio <0.7 or the lower limit of normal (LLN) for the diagnosis of COPD. GOLD favors the use of the former because it is simple and independent of reference values, since it relates to variables measured in the same individual and has been used in all the clinical trials that form the evidence base on which treatment recommendations are drawn. GOLD 2023 acknowledges that use of a fixed FEV1/FVC ratio < 0.7 to define airflow obstruction may underdiagnose young adults and over-diagnose the elderly[55,56], especially in mild disease, compared to using the LLN values of FEV1/FVC. LLN values are based on the normal distribution and classify the bottom 5% of the healthy population as abnormal. Thus, LLN values are highly dependent on the choice of reference equations as well as race/ethnicity, and there are no longitudinal studies available validating the use of the LLN. Further, using the fixed ratio is not inferior to LLN regarding prognosis[57]. Finally, the risk of misdiagnosis and over-treatment of individual patients using the fixed ratio as a diagnostic criterion is limited, as spirometry is only one biologic measurement to establish the clinical diagnosis of COPD in the appropriate clinical context (symptoms and risk factors). Diagnostic simplicity and consistency are crucial for the busy clinician.
Fourth, while post-bronchodilator spirometry is required for the diagnosis and assessment of COPD, assessing the degree of reversibility of airflow obstruction to inform therapeutic decisions is no longer recommended[58]. The degree of reversibility in a single patient varies over time and has not been shown to differentiate COPD from asthma (except when airflow limitation disappears following bronchodilators, which is incompatible with COPD), or to predict the response to long-term treatment with bronchodilators or corticosteroids[59]. Accordingly, it is not necessary nor advised[1] to stop inhaled medication before obtaining spirometry measurements during follow-up of patients.
Finally, the role of screening spirometry in the general population for the diagnosis of COPD is also controversial. In asymptomatic individuals without any significant exposure to tobacco or other risk factors, screening spirometry is probably not indicated. By contrast, in those with symptoms and/or risk factors (e.g., > 20 pack-years of smoking, recurrent chest infections, prematurity or other significant early life events), the diagnostic yield for COPD is relatively high and spirometry should be considered as a method for case finding[1].
TERMINOLOGY
As mentioned above, it is now well established that a range of lung function trajectories exist through life[28,60] and that COPD can develop by both abnormal lung development and/or accelerated lung aging[42]. This has generated some terminological confusion, so GOLD proposes use of the following terminology[1]:
Early COPD
The word “early” means “near the beginning of a process”. Because COPD can start early in life and take a long time to manifest clinically, identifying “early” COPD is difficult. Further, a biological “early” related to the initial mechanisms that eventually lead to COPD should be differentiated from a clinical “early”, which reflects the initial perception of symptoms, functional limitation and/or structural abnormalities noted. Thus, GOLD proposes to use the term “early COPD” only to discuss the “biological” first steps of the disease in an experimental setting.
Mild COPD
Some studies have used “mild” airflow obstruction as a surrogate for “early” disease[61]. This assumption is incorrect because not all patients started their journey from a normal peak lung function in early adulthood, so some of them may never suffer “mild” disease in terms of “severity” of airflow obstruction[28]. Further, “mild” disease can occur at any age and may progress, or not, over time[60]. Accordingly, GOLD proposes that “mild” should be used only to describe the severity of airflow obstruction measured spirometrically.
YOUNG COPD
The term “young COPD” is seemingly straightforward because it directly relates to the “chronological” age of the patient[62]. Given that lung function peaks at around 20 years[5] and starts to decline around 40-50 years, GOLD proposes to operationally consider “young COPD” in patients aged 20–50 years[63], whether from having never achieved normal peak lung function in early adulthood and/or from shorter plateau and/or early lung function decline[64,65]. COPD in “young” people may be associated with significant structural and functional lung abnormalities with substantial impact on health[65,66]. A family history of respiratory diseases and/or early-life events (including hospitalizations before the age of 5 years) is reported by a significant proportion of young patients with COPD[65].
PRE-COPD
This term has been proposed to identify individuals of any age, with respiratory symptoms and/or other detectable structural (e.g., emphysema) and/or functional abnormalities (e.g., hyperinflation, reduced lung diffusing capacity, or rapid FEV1 decline), in the absence of airflow obstruction on post-bronchodilator spirometry (i.e., FEV1/FVC>0.7)[67]. These patients may (or not) develop persistent airflow obstruction (i.e., COPD) over time[67]. Yet, people with pre-COPD so defined should already be considered “patients” because they suffer symptoms and/or have functional and/or structural abnormalities. Currently, there is no evidence on what the best treatment is for these patients[68]. There urgently is a need for RCTs, both in patients with ‘Pre-COPD’, and in young people with COPD[69]. Research in this area would benefit from pediatric-to-adulthood cohorts and more active case-finding strategies.
PRISm
This term has been proposed to describe individuals with FEV1/FVC ≥ 0.7 and FEV1 < 80% of reference after bronchodilation[70,71]. Its prevalence ranges from 7.1% to 20.3%[71], is particularly high in current and former smokers, and is associated with increased all-cause mortality[71]. PRISm can transition to normal, obstructive or restrictive spirometry over time[71]. Despite an increasing body of literature on PRISm, significant knowledge gaps remain in relation to its pathogenesis and treatment[71].
TAXONOMY
Based on the different causes (or etiotypes) that can contribute to COPD (see above) GOLD 2023 proposes a new taxonomic classification of COPD [Figure 1] that reflects two recent proposals[2,72]. It aims to raise awareness about non-smoking related COPD and to stimulate research on the mechanisms and corresponding diagnostic, preventive or therapeutic approaches for these other etiotypes of COPD which are highly prevalent around the globe[13].
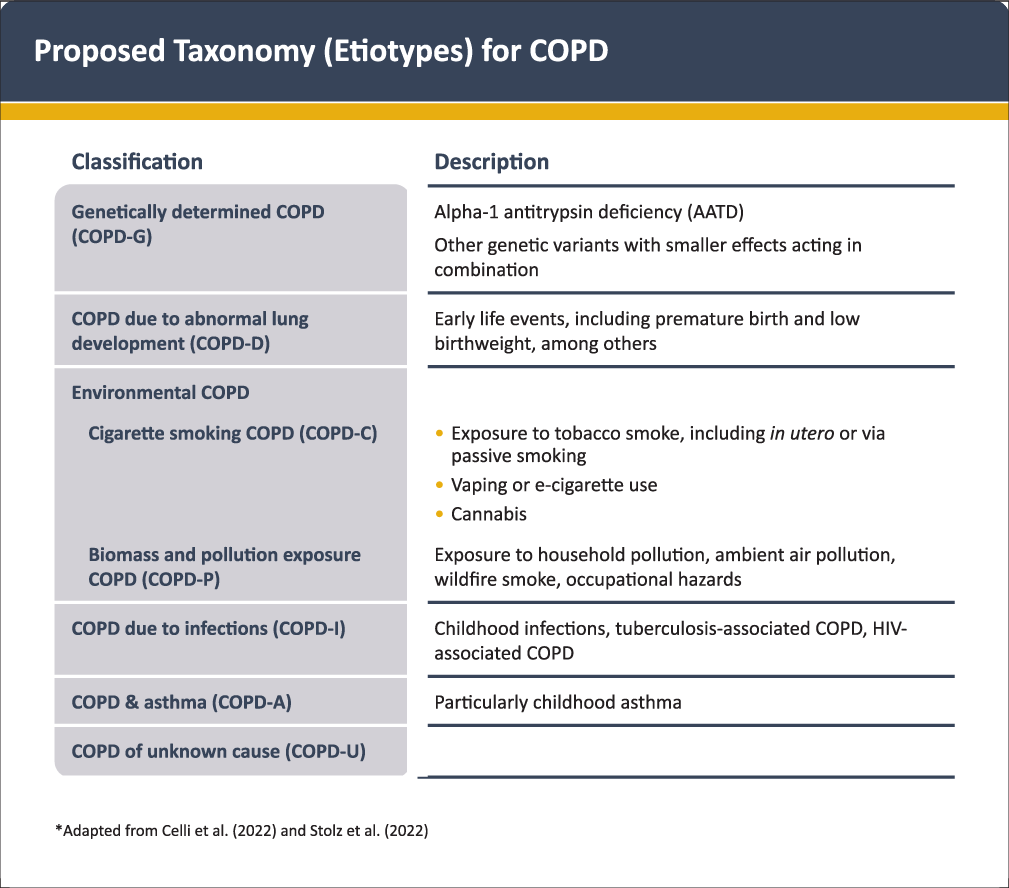
- Proposed taxonomy (etiotypes) for COPD. Reproduced with permission from www.goldcopd.org.
CLINICAL PRESENTATION
Patients with COPD may complain of dyspnea, wheezing, chest tightness, fatigue, activity limitation and/or cough with or without sputum production and may experience acute respiratory events characterized by acute worsening of respiratory symptoms called exacerbations that require specific preventive and therapeutic measures. Patients with COPD frequently harbor other comorbid diseases (multimorbidity) that influence their clinical condition and prognosis[73], independently of the severity of airflow obstruction due to COPD[73], and require specific treatment (see below).
ASSESSMENT
Once the diagnosis of COPD has been confirmed by spirometry, the goals of the initial assessment of COPD to guide therapy are to determine: (1) the severity of airflow limitation (GOLD spirometric grades); (2) the nature and magnitude of current symptoms; (3) the previous history of moderate and severe exacerbations (the best estimate of the risk of future exacerbations); and (4) the presence and type of multimorbidity.
Combined initial COPD assessment: from ABCD to ABE
GOLD 2023 modifies the ABCD assessment tool of previous editions[74] to recognize the clinical impact of exacerbations independently of the level of symptoms of the patient[75] [Figure 2]. The thresholds proposed for symptoms (X axis) and history of exacerbations in the previous year (Y axis) are unchanged from previous GOLD documents, so the A and B groups remain unchanged, while the former C and D groups are now merged into a single group termed “E” (for “Exacerbations”). This has implications for the initial pharmacological treatment recommendations, as discussed below. The practical value of this proposal needs to be validated by appropriate clinical research.

- GOLD ABE assessment tool. Exacerbation history refers to exacerbations suffered the previous year. mMRC: modified Medical Research Dyspnea Questionnaire. CAT: COPD Assessment Test. Reproduced with permission from www.goldcopd.org.
Imaging
A chest X-ray cannot confirm a diagnosis of COPD. However, radiological changes associated with COPD may include signs of lung hyperinflation (flattened diaphragm and increased retrosternal air space), lung hyperlucency, and rapid tapering of the vascular markings. On the other hand, a chest X-ray can help exclude alternative diagnoses and establishing the presence of significant comorbidities such as concomitant pulmonary fibrosis, bronchiectasis, pleural diseases, kyphoscoliosis, and cardiomegaly.
CT of the chest can provide information of potential clinical relevance, including: (1) presence, severity, and distribution of emphysema. This has implications for potential surgical or endoscopic lung volume reduction, is associated with faster FEV1 decline, higher mortality and increased risk of lung cancer[76]; (2) about 30% of COPD patients have bronchiectasis visible on CT, which are associated with increased exacerbation frequency and mortality[77]; (3) most COPD patients fulfill the inclusion/exclusion criteria for lung cancer screening in the general population[78,79], so they should be offered a similar strategy[1]; (4) quantification of airway abnormalities, although these methods are less well standardized than those used for emphysema quantification[80-82]; and, (5) a CT offers information about COPD comorbidities including coronary artery calcifications, pulmonary artery enlargement, bone density and muscle mass, some of which are associated with all-cause mortality independently of the severity of airflow obstruction[83]. Thus, GOLD 2023 recommends chest CT in COPD patients with persistent exacerbations, symptoms out of proportion to airflow limitation severity, severe airflow obstruction with significant hyperinflation and gas trapping, or for those who meet criteria for lung cancer screening.
PHARMACOLOGICAL TREATMENT
Pharmacological therapy must be always associated with non-pharmacological measures described later, starting with smoking cessation when needed.
Choice and appropriate use of inhaler devices
Because inhaled therapy is the cornerstone of COPD treatment, the appropriate use of these devices is crucial to optimize the benefit-risk ratio of any inhaled therapy. Achieving this goal requires educating and training the providers and the patients in the correct use of the device. Regular assessment at follow-up is necessary to maintain their effective use. Details on the choice of device can be found in the complete GOLD 2023 document and include availability, patient preferences and ability to perform the correct inhalation maneuver[84].
Initial pharmacological treatment
Figure 3 shows the 2023 GOLD recommendation for initiation of pharmacological therapy. The treatment of patients in Group A has not changed. In contrast, for patients in Group B, a dual long-acting bronchodilator combination (β2 adrenergic (LABA) + anti-muscarinic (LAMA) bronchodilators] is now recommended since dual therapy is more effective than monotherapy with similar side-effects[85-87]. For patients in Group E, LAMA+LABA is also the recommended initial therapy, except for those patients with blood eosinophils ≥ 300 cells/µL, in whom starting triple therapy (LABA+LAMA+ICS) can be considered. This is a practical recommendation; direct evidence is not available to guide therapy in naïve individuals. The role of the blood eosinophil count for the reduction of the exacerbation risk with ICS is explicitly discussed below. The use of LABA+ICS in COPD is no longer encouraged. If there is an indication for an ICS, then LABA+LAMA+ICS has been shown to be superior to LABA+ICS and is therefore the preferred choice[88,89]. If patients with COPD have concomitant asthma, they should be treated as if they have asthma[90].
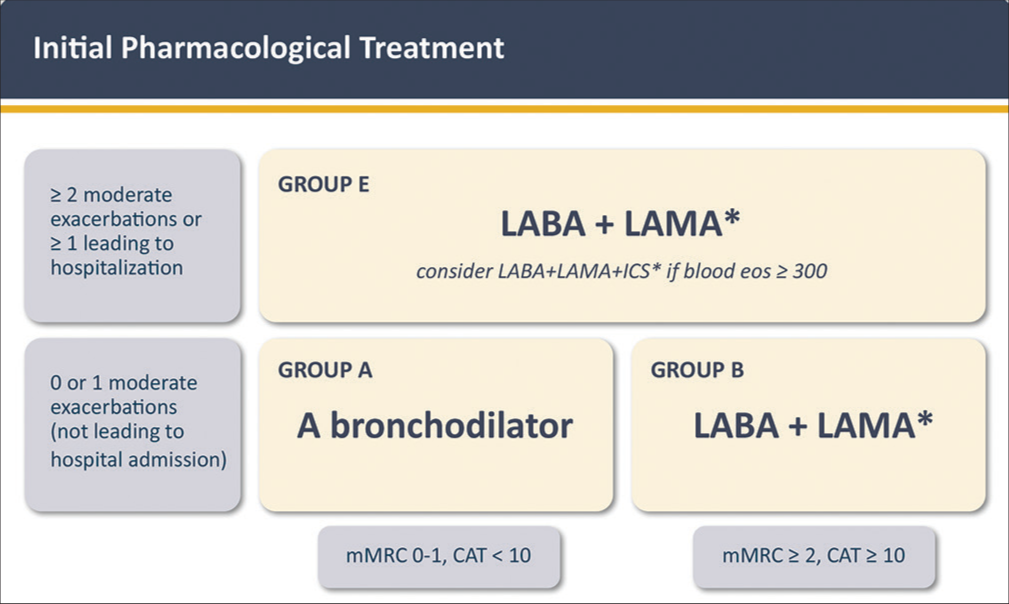
- Initial pharmacological treatment. Exacerbation history refers to exacerbations suffered the previous year. *: single inhaler therapy may be more convenient and effective than multiple inhalers. mMRC: modified Medical Research Dyspnea Questionnaire. CAT: COPD Assessment Test. LAMA: long-acting anti-muscarinic antagonist; LABA: long-acting β2 receptor agonist; ICS: inhaled corticosteroid; eos: eosinophils. Reproduced with permission from www.goldcopd.org.
Follow-up pharmacological treatment
Following initial therapy, patients should be reassessed guided by the principles of first review and assess, then adjust if needed.
GOLD 2023 continues to recommend that follow-up treatment be based on two key treatable traits (TTs)[91]: dyspnea and occurrence of exacerbations [Figure 4]. TTs can be identified based on clinical recognition (phenotypes) and/or on deep understanding of critical causal pathways (endotypes) through validated biomarkers (e.g., circulating eosinophils to guide treatment with inhaled corticosteroids (ICS) in COPD patients with evidence of T2 inflammation)[91]. Importantly, TTs can co-exist in the same patient[92] and change with time (spontaneously or because of treatment). GOLD 2023 recommendations for follow-up treatment for both TTs (dyspnea and exacerbations) broadly follow previous recommendations but no longer include LABA+ICS for the reasons stated above (see initial treatment).
For patients with persistent dyspnea or exercise limitation on bronchodilator monotherapy, a step up to LABA+LAMA is recommended. If this does not improve symptoms clinicians should consider switching inhaler device or molecules, as well as investigating and treating other causes of dyspnea.
For patients continuing to have exacerbations (with or without dyspnea) on bronchodilator monotherapy, escalation to LABA+LAMA is recommended, except for patients with blood eosinophils ≥ 300 cells/µL who may be escalated to LABA+LAMA+ICS. For patients with persistent exacerbations on LABA+LAMA, escalation to LABA+LAMA+ICS is recommended if they have blood eosinophils ≥ 100 cells/µL. For patients continuing to exacerbate despite therapy with LABA+LAMA+ICS or those who have an eosinophil count of < 100 cells/µL, the addition of roflumilast (particularly in patients with chronic bronchitis and an FEV1 < 50% predicted)[93-95] or a macrolide (particularly in patients who are not current smokers) may be considered[96,97].
Patients whose pharmacological treatment is modified should be closely monitored. Treatment escalation has not been systematically tested and trials of de-escalation are limited to withdrawing ICS[98]. As indicated in Figure 4, ICS de-escalation can be considered if pneumonia or other considerable side-effects. In case of blood eos≥300 cells/µl, ICS de-escalation is more likely to be associated with development of exacerbations. Finally, if a patient with COPD and no features of asthma has already been treated – for whatever reason – with LABA+ICS and is well controlled in terms of symptoms and exacerbations, then LABA+ICS could be continued. However, if they remain dyspneic switching to LABA+LAMA should be considered, and if they have further exacerbations, treatment should be escalated to LABA+LAMA+ICS.

- Follow-up pharmacological treatment. *: single inhaler therapy may be more convenient and effective than multiple inhalers; **: Consider de-escalation of ICS if pneumonia or other considerable side-effects. In case of blood eos ≥300 cells/µl de-escalation is more likely to be associated with the development of exacerbations. Exacerbation history refers to exacerbations suffered the previous year. mMRC: modified Medical Research Dyspnea Questionnaire. CAT: COPD Assessment Test. LAMA: long-acting anti-muscarinic antagonist; LABA: long-acting β2 receptor agonist; ICS: inhaled corticosteroid; eos: eosinophils. Reproduced with permission from www.goldcopd.org.
Other therapeutic considerations
The eosinophil as a useful clinical biomarker
As in previous GOLD reports, the main factors to consider whether to initiate ICS treatment is based on a patient’s previous exacerbation history, and the blood eosinophil count [Figure 5][88,89,99-102]. Adding ICS has little or no effect at a blood eosinophil count < 100 cells/µL whilst blood eosinophils ≥ 300 cells/µL identify patients with a strong likelihood of treatment benefit[103,104]. There is a continuous gradation of the preventive effect of ICS in patients with eosinophil values between 100 and 300 cells/µL, so some patients are likely to get benefit from adding ICS[103,104]. Treatment decisions can be based on historical eosinophil counts as the repeatability of blood eosinophil counts in a large primary care population appears reasonable[105], although greater variability is observed at higher thresholds[106].

- Factors to consider when adding treatment with inhaled corticosteroids (ICS) to long-acting bronchodilators (note the scenario is different when considering ICS withdrawal). *: despite appropriate long-acting bronchodilator maintenance therapy; # note that blood eosinophils should be seen as a continuum; quoted values represent approximate cut-points; eosinophil counts are likely to fluctuate. Reproduced with permission from www.goldcopd.org.
Chronic bronchitis
Chronic Bronchitis (CB) has been traditionally defined by “cough and sputum production for at least 3 months per year for two consecutive years” (in the absence of another cause that can explain this, a caveat that is often forgotten). The prevalence of CB in COPD patients ranges from 27-35%, being higher in males, younger age, greater pack-years of smoking, more severe airflow obstruction, rural location and increased occupational exposures. CB is associated with accelerated lung function decline, exacerbations, and mortality in COPD patients. Treatment of CB is unresolved but can include smoking cessation, long-acting muscarinic antagonists, oral mucolytics and antioxidants or oscillating positive expiratory pressure therapy; the use of inhaled mucolytics or recombinant human DNase have not shown promise[1]. Liquid nitrogen metered cryospray, rheoplasty, and targeted lung denervation are currently undergoing evaluation for CB treatment.
Affordability of inhaled medicines
In LMICs, there is limited availability and affordability of essential inhaled therapies for people with COPD, and this global inequity must be addressed urgently as part of efforts to achieve Universal Health Coverage and Sustainable Development Goal 3[107]. On the other hand, even in developed countries, most inhaled medicines are still branded.
Reducing lung function decline and mortality in COPD
Pharmacotherapy has the potential to reduce the rate of lung function decline, but further studies are needed to know what patients can benefit most since not all patients exhibit accelerated lung function decline[1]. On the other hand, a number of pharmacologic and non-pharmacologic interventions [Figure 6] reduce mortality in selected COPD patients. This emphasizes the need to implement targeted case-finding strategies, apply adequate patient characterization and provide appropriately individualized therapy.
![Evidence supporting a reduction in mortality with pharmacotherapy and non-pharmacotherapy in COPD patients. *: RCT with pre-specified analysis of the mortality outcome (primary or secondary outcome. + Not conclusive results likely due to differences in PR across a wide range of participants and settings. Definition of abbreviations: ICS: inhaled corticosteroids; LABA: long-acting β2-agonist; LAMA: long acting anti-muscarinic. Reference correspondence: 1.[89,192]; 2.[193]; 3.[156,157,194]; 4.[195,196]; 5.[197]; 6.[198]. Reproduced with permission from www.goldcopd.org.](/content/124/2024/5/3/img/JPATS-5-092-g006.png)
- Evidence supporting a reduction in mortality with pharmacotherapy and non-pharmacotherapy in COPD patients. *: RCT with pre-specified analysis of the mortality outcome (primary or secondary outcome. + Not conclusive results likely due to differences in PR across a wide range of participants and settings. Definition of abbreviations: ICS: inhaled corticosteroids; LABA: long-acting β2-agonist; LAMA: long acting anti-muscarinic. Reference correspondence: 1.[89,192]; 2.[193]; 3.[156,157,194]; 4.[195,196]; 5.[197]; 6.[198]. Reproduced with permission from www.goldcopd.org.
NON-PHARMACOLOGICAL THERAPY
Non-pharmacological treatment is a key part of the comprehensive management of COPD.
Education
All patients should receive basic information about COPD and its treatment [respiratory medications and inhalation devices), strategies to minimize dyspnea, and advice about when to seek help.
Smoking cessation
Approximately 40% of people with COPD continue to smoke despite knowing they have the disease, and this behavior has a negative impact on prognosis and progression of the disease[108]. All patients who continue to smoke should be offered help and treatment to quit.
Vaccination
Depending on local guidelines, patients should be offered vaccination against influenza, pneumococcus, COVlD-19, pertussis, herpes zoster, if they have not already received these[1].
PHYSICAL ACTIVITY
Physical activity is decreased in COPD patients[109] so all COPD patients should be encouraged to keep active. The challenge is promoting and maintaining physical activity[110,111]. Technology-based interventions have the potential to provide convenient and accessible means to enhance exercise self-efficacy, and to educate and motivate patients to make healthy lifestyle changes[112].
Pulmonary Rehabilitation
Pulmonary Rehabilitation (PR), including community and home-based, is beneficial[1]. Accordingly, patients with high symptom burden and risk of exacerbations (GOLD groups B and E) should be recommended to take part in a formal PR program designed and delivered in a structured manner, considering the individual’s COPD characteristics and comorbidities[113-116].
Tele-rehabilitation has been proposed as an alternative to the traditional approaches. This has become even more relevant in the COVID-19 pandemic era where in-person PR has not been feasible. However, it is important to distinguish between evidence-based tele-rehabilitation models and pandemic-adapted models. Multiple trials performed in groups and individuals with a large variety of tele-rehabilitation delivery platforms (videoconferencing, telephone only, website with telephone support, mobile application with feedback, centralized “hub” for people to come together) suggest that telerehabilitation is safe and has similar benefits to those of center-based PR across a range of outcomes[117]. However, the optimal form of delivery, content and duration are not yet established[118,119].
Oxygen therapy & Ventilatory Support
The criteria for prescribing long term oxygen therapy and ventilator support remain unchanged and are described in detail in the GOLD 2023 report[1].
Surgical and endoscopic lung volume reduction
In selected patients with symptomatic heterogeneous or homogenous emphysema and significant hyperinflation refractory to optimized medical care, surgical or bronchoscopic modes of lung volume reduction may be considered [Figure 7]. In patients with a large bulla, surgical bullectomy is an option, and in selected patients with very severe COPD and without relevant contraindications, lung transplantation may be considered.

- Surgical and interventional therapies in advanced emphysema. Note: not all therapies are available in all countries. Long term ELVR outcomes or direct comparisons to LVRS are unknown. Homogeneous emphysema was defined as < 10% difference in emphysematous destruction between the targeted and ipsilateral non-targeted lobe undergoing lung reduction as measured by quantitative Chest CT imaging. By contrast, greater than 10% difference between the targeted and non-targeted lobe is considered a heterogeneous pattern of emphysematous destruction. Definition of abbreviations: CV: collateral ventilation measure by Chartis; FI+: fissure integrity >90% by HRCT; FI-: fissure integrity<90% by HRCT; ELVR: Endoscopic Lung Volume Reduction; EBV: Endobronchial Valve; VA: Vapor ablation; LVRC: Lung Volume Reduction Coil; LVRS: Lung Volume Reduction Surgery. Reproduced with permission from www.goldcopd.org.
End of Life and Palliative Care
All patients with advanced COPD should be considered for end of life and palliative care support to optimize symptom control and allow patients and their families to make informed choices about future management.
EXACERBATIONS OF COPD
A new definition
Exacerbations of COPD (ECOPD) negatively impact health status, disease progression and prognosis[120]. The previous GOLD definition of ECOPD was highly non-specific (“acute worsening of respiratory symptoms that results in additional therapy”)[3].
Besides, the severity of ECOPD was determined post facto (mild, moderate or severe) based on the use of healthcare resources[3], which is useless to guide treatment at the point of care.
To address these limitations, GOLD 2023 has adopted the recent consensus Rome proposal[120] which defines ECOPD as: “an event characterized by dyspnea and/or cough and sputum that worsen over ≤14 days, which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and systemic inflammation caused by airway infection, pollution, or other insult to the airways”.
Differential Diagnosis
Patients with COPD are at increased risk of other acute events, particularly decompensated heart failure, pneumonia and/or pulmonary embolism that may mimic or aggravate an ECOPD [Figure 8][121]. Thus, while worsening of dyspnea, particularly if associated with cough and, purulent sputum, and no other symptoms or signs in a patient with COPD may be diagnosed as an ECOPD, other patients may have worsening of respiratory symptoms, particularly dyspnea without the classic characteristics of ECOPD, that should prompt careful consideration and/or search of those potential confounders, or contributors[121].
ASSESSMENT OF ECOPD SEVERITY
Based on a thorough review of the available literature and using a Delphi approach to agree on the variable thresholds, the Rome proposal suggests using easy to obtain clinical variables to define the severity of ECOPD (mild, moderate or severe) at the point of care [Figure 8][120]. In the primary care setting, severity can be determined with the easily obtainable dyspnea intensity (using a VAS 0 to 10 dyspnea scale with zero being not short of breath at all and 10 the worst shortness of breath you have ever experienced), respiratory rate, heart rate and oxygen saturation level. Where available, measuring blood C-reactive protein (CRP) levels is recommended [Figure 8]. To determine the need for ventilatory support (usually in the emergency room or hospital setting) arterial blood gases should be measured. To move from a mild to a moderate level, three of the variables need to exceed the established thresholds.
![Classification of the severity of COPD exacerbations. Definition of abbreviations: VAS: visual analog scale; RR: respiratory rate; HR: heart rate; CRP: C-reactive protein. SaO2: arterial oxygen saturation; PaO2: arterial partial pressure of oxygen; ABG: arterial blood gases; ABG should show new onset/worsening hypercapnia or acidosis since a few patients may have chronic hypercapnia. Adapted from ref.[120]. Reproduced with permission from www.goldcopd.org.](/content/124/2024/5/3/img/JPATS-5-092-g008.png)
- Classification of the severity of COPD exacerbations. Definition of abbreviations: VAS: visual analog scale; RR: respiratory rate; HR: heart rate; CRP: C-reactive protein. SaO2: arterial oxygen saturation; PaO2: arterial partial pressure of oxygen; ABG: arterial blood gases; ABG should show new onset/worsening hypercapnia or acidosis since a few patients may have chronic hypercapnia. Adapted from ref.[120]. Reproduced with permission from www.goldcopd.org.
It is hoped that prospective validation will help better define exacerbations and their severity at point of contact, and that documented validation may confirm or help modify the proposed thresholds of the variables now included[122]. Likewise, it is proposed that prospective research can help determine a more specific marker of lung injury than the more generic CRP, as has been true for acute events of other organs (e.g., troponin level in patients with acute myocardial infarction).
Management of ECOPD
Treatment setting
Depending on the episode severity, as well as that of the underlying COPD and comorbidities, an ECOPD can be managed in either the outpatient or inpatient setting. The following are indications for hospitalization: (1) severe symptoms such as sudden worsening of resting dyspnea, high respiratory rate, decreased oxygen saturation, confusion, drowsiness; (2) acute respiratory failure; (3) onset of new physical signs (e.g., cyanosis, peripheral edema); (4) failure to respond to initial medical management; (5) presence of serious comorbidities (e.g., heart failure, newly occurring arrhythmias, etc.); and, (6) insufficient home support[1].
In the emergency department, hypoxemic patients should be provided with the appropriate concentration of supplemental oxygen and be assessed to determine if the increased work of breathing or impaired gas exchange require non-invasive ventilation. If so, healthcare providers should consider admission to an area where proper monitoring and care can be provided. In less severe cases, the patient may be managed in the emergency department or hospital ward unit.
Pharmacological treatment
Bronchodilators
Short-acting inhaled β2-agonists (SABA), with or without short-acting anticholinergics (SAMA), are the initial bronchodilators for acute treatment of ECOPD, administered using a metered-dose inhaler (MDI, with a spacer device if necessary, or nebulization[1]. If a nebulizer is chosen, air-driven is preferable to oxygen-driven nebulization to avoid the potential risk of increasing PaCO2[123]. The GOLD 2023 report recommends continuing treatments with long-acting bronchodilators during the exacerbation or to start these medications as soon as possible before hospital discharge[1]. Intravenous methylxanthines (theophylline or aminophylline) are not recommended due to lack of efficacy and significant side effects[124,125].
Glucocorticoids
Systemic glucocorticoids in COPD exacerbations improve lung function, oxygenation, risk of early relapse, and reduce treatment failures and length of hospitalization[126-128]. A dose of 40 mg prednisone-equivalent per day for 5 days is recommended[129]. Longer courses increase risk of pneumonia and mortality[130]. Therapy with oral prednisolone is equally effective to intravenous administration[131]. Nebulized budesonide may be a suitable alternative to systemic corticosteroids in some patients[127,132]. Recent studies suggest that glucocorticoids may be less efficacious to treat COPD exacerbations in patients with lower blood eosinophil levels[133].
Antibiotics
Antibiotics should be given to patients with ECOPD who have increased sputum volume and sputum purulence and most of those requiring mechanical ventilation (invasive or noninvasive)[134]. The recommended length of antibiotic therapy is 5-7 days[135]. The choice of the antibiotic should be based on the local bacterial resistance pattern. Usually, initial empirical treatment is an aminopenicillin with clavulanic acid, macrolide, tetracycline or, in selected patients, quinolone. In patients with frequent exacerbations, severe airflow obstruction and/or exacerbations requiring mechanical ventilation, cultures from sputum or other materials from the lung should be performed, as gram-negative bacteria (e.g., Pseudomonas species) or resistant pathogens that are not sensitive to the above-mentioned antibiotics may be present. The route of administration (oral or intravenous) depends on the patient’s ability to ingest medications and the pharmacokinetics of the antibiotic.
Adjunct therapies
Additional therapies may be indicated to maintain appropriate fluid balance, treat the comorbidities and monitor nutritional aspects. Hospitalized patients with COPD are at an increased risk of deep vein thrombosis and pulmonary embolism and prophylactic measures for thromboembolism should be instituted[136,137]. At all times, healthcare providers should strongly enforce the need for smoking cessation.
Oxygen therapy
Supplemental oxygen for hypoxemia should be titrated to a target saturation of 88-92%[138]. In severe ECOPD, blood gases should be checked frequently or as clinically indicated to monitor for carbon dioxide retention and/or worsening acidosis. Pulse oximetry is not as accurate as arterial blood gas measurement[139] and, in particular, may overestimate blood oxygen content among individuals with darker skin tones[140]. Venturi masks offer more accurate and controlled delivery of inspired oxygen than do nasal prongs[1].
High-flow nasal therapy (HFNT) delivers heated and humidified air-oxygen blends via special devices at rates up to 8 L/min in infants and up to 60 L/min in adults[141]. HFNT has been associated with decreased respiratory rate and effort, improved lung mechanics and gas exchange, prolonged time to next exacerbation and improved health-related quality of life scores in COPD patients with acute (or chronic) hypercapnia[142-144], but did not prevent intubation in hospitalized patients with ECOPD[145]. In fact, the European Respiratory Society (ERS) recommends ventilatory support before using HFNT in hypercapnic ECOPD[146].
Ventilatory support
Ventilatory support can be provided by either noninvasive (NIV) means, with a nasal or facial mask, or invasive means, with an oro-tracheal tube or tracheostomy ventilation. NIV is the preferred initial mode of ventilation[147,148]. It improves gas exchange and decreases respiratory rate, work of breathing, severity of breathlessness, intubation rates, complications (e.g., ventilator associated pneumonia), length of hospital stay and mortality[147,148]. Once patients improve and can tolerate at least 4 hours of unassisted breathing, NIV can be directly discontinued without any need for a “weaning” period[149].
Patients who fail non-invasive ventilation should receive invasive ventilation as subsequent rescue therapy[150]. The use of invasive ventilation in COPD is influenced by the likely reversibility of the precipitating event, the patient’s wishes, and the availability of intensive care facilities[150]. When possible advance directives or “living will”, makes these difficult decisions easier to resolve. Major hazards include the risk of ventilator-acquired pneumonia, barotrauma and volutrauma, and the risk of tracheostomy and consequential prolonged ventilation. Respiratory stimulants (e.g., caffeine, doxapram) are not recommended to treat ECOPD[1].
Hospital discharge, early readmissions, and follow-up
There are no standards to the timing and nature of hospital discharge but early readmissions during the first 90 days after discharge are frequent and constitute a significant health care problem. A systematic review has shown that comorbidities, previous exacerbations and hospitalization, and increased length of stay were significant risk factors for 30- and 90-day all-cause readmission after a hospitalized COPD exacerbation[151]. Hence, after an exacerbation it is good practice to cover education on correct use of the medications, provide support at home and a follow-up plan before discharge[152]. Early follow-up (within one month) should also be scheduled as it has been related to less exacerbation-related readmissions[153]. Additional follow-up at three months is recommended to ensure return to a stable clinical state and permit a review of the patient’s symptoms, lung function, and where possible the assessment of prognosis using multiple scoring systems such as the BODE[154]. In addition, arterial oxygen saturation and arterial blood gas assessment will determine the need for long-term oxygen therapy more accurately[155]. The effects of initiation of pulmonary rehabilitation in the first 4 weeks post hospital discharge are unclear[156,157].
Prognosis
Long-term prognosis following hospitalization for COPD exacerbation is poor, with a five-year mortality rate of about 50%[158]. Factors independently associated with poor outcome include older age, lower BMI, comorbidities (e.g., cardiovascular disease or lung cancer), previous hospitalizations for COPD exacerbations, clinical severity of the index exacerbation and need for long-term oxygen therapy at discharge[159-161].
COMORBIDITIES, MULTIMORBIDITY AND FRAILTY
COPD almost invariably coexists with other diseases (see below) that may significantly impact the patient’s clinical condition and prognosis[162] These comorbid conditions complicate the clinical picture because they can mimic the clinical presentation of COPD with similar complaints of dyspnea and chest tightness/pain and lead to misdiagnosis and missed opportunities for treatment. Additionally, comorbid conditions can further limit the pulmonary reserve of patients with COPD. Conversely, COPD may adversely affect the outcomes of many other disorders. For example, patients with heart failure or those undergoing coronary artery bypass grafting have greater morbidity and mortality when COPD is present compared to when it is absent. Some comorbidities arise independently of COPD, but others are causally related, either by shared risk factors or by one disease compounding the severity of the other[163].
In general, the presence of comorbidities should not alter COPD treatment and comorbidities should be treated per usual standards regardless of the presence of COPD. Attention should be directed to ensure simplicity of treatment and to minimize polypharmacy.
Cardiovascular Diseases are common in COPD, their prevalence ranging from 20 to 70%[164]. The types of cardiovascular comorbid conditions are diverse and span the spectrum from congestive heart failure to ischemic heart disease, arrythmias, peripheral vascular disease and hypertension[164]. All these conditions should be treated in patients per established guidelines independent of the COPD diagnosis, including the use of selective β1-blockers when a clear cardiovascular indication is present.
Lung cancer occurs frequently in patients with COPD[165]. Like the general population, annual low-dose CT scan is recommended for lung cancer screening in COPD due to smoking[78,79]. In patients with COPD not due to smoking, there is insufficient data to establish benefit over harm from lung cancer screening.
Bronchiectasis affects approximately 30% of patients with COPD[166]. Increased sputum production, recurrent infections, and more frequent exacerbations hallmark bronchiectasis when it accompanies COPD. A chest CT scan is recommended if bronchiectasis is suspected.
Sleep apnea occurs in approximately 14% of COPD patients[167]. This worsens their prognosis since they have more frequent episodes of oxygen desaturation and a longer sleep time with hypoxemia and hypercapnia than OSA patients without COPD.
Osteoporosis Osteoporosis in COPD is often under-diagnosed and associated with poor health status and prognosis[168]. Recurrent use of systemic corticosteroids increases the risk of osteoporosis and should be avoided if possible.
Diabetes and metabolic syndrome. Studies show that diabetes is more frequent in COPD and the latter is likely to affect prognosis[164]. The prevalence of metabolic syndrome has been estimated to be more than 30%[169]. Diabetes and metabolic syndrome should be treated according to usual guidelines. COPD should be treated as usual.
Gastroesophageal reflux (GERD) GERD is an independent risk factor for exacerbations and is associated with worse health status[170,171]. The mechanisms responsible for increased risk of exacerbations are not yet fully established. Proton pump inhibitors are often used for treatment of GERD. One small, single-blind study suggested that these agents decrease the risk of exacerbation[172], but their value in preventing these events remains controversial, and the most effective treatment for this condition in COPD has yet to be established[173].
Anemia is frequent in patients with COPD[174]. These patients are generally older, have more frequent cardiometabolic comorbidities, greater dyspnea, worse quality of life and airflow obstruction, reduced exercise capacity, and an increased risk of severe exacerbations with a higher mortality.
Secondary polycythemia may be associated with pulmonary hypertension[175,176] venous thromboembolism[176] and mortality. Although the prevalence of polycythemia in COPD has decreased following the introduction of long-term oxygen therapy (LTOT)[177], one study reported its presence in 8.4% of patients with severe COPD receiving LTOT[178].
Mental health Anxiety and depression are important and underdiagnosed comorbidities in COPD[179-182]. Both are associated with poor prognosis[181,183], younger age, female sex, smoking, lower FEV1, cough, higher SGRQ score, and a previous history of cardiovascular disease[179,182,184]. Cognitive impairment occurs in 32% of people with COPD, but neuropsychological testing suggests that up to 56% of them may suffer from it[185,186].
Multimorbidity and frailty An increasing number of aging patients suffer multi-morbidity, defined as the presence of two or more chronic conditions. These patients have symptoms from multiple diseases making their presentation complex in the acute or chronic state. Multimorbidity results in frailty hallmarked by the presence of weakness, fatigue, exhaustion, low physical activity, and unintentional weight loss[187], a condition that has been reported to be more prevalent in patients with COPD.
COPD AND COVID-19
Patients should follow basic infection control measures to help prevent SARS-CoV-2 infection including social distancing and washing hands which are associated with reductions in the incidence COVID-19[188]. They should have COVID-19 vaccination in line with national guidelines. At times of high community incidence of COVID-19, patients should be advised to wear a facial covering[1] and should keep taking their oral and inhaled respiratory medications for COPD as directed as there is no evidence that COPD medications should be changed during this COVID-19 pandemic[189].
Marked reductions in exacerbation rates and hospitalization for COPD have been reported during the initial phases of the pandemic[190], possibly because of infection control measures. Physical distancing and shielding, or sheltering-in-place, should not lead to social isolation and inactivity. Patients should stay in contact with their friends and families by telecommunication and continue to keep active. They should also ensure they have enough medication.
COPD patients are not at increased risk of infection with SARS-CoV-2, but this may reflect the effect of protective strategies[189,191]. After accounting for potential confounding variables, COPD patients do have a higher risk of hospitalization, ICU admission, and mortality[191]. COPD patients presenting with new or worsening respiratory or other symptoms that could be COVID-19 related, even if these are mild, should be tested for SARS-CoV-2.
NEW OPPORTUNITIES
COPD is a common, preventable, and treatable disease, but extensive under-diagnosis and misdiagnosis leads to patients receiving no treatment or incorrect treatment[1]. The realization that environmental factors other than tobacco smoking can contribute to COPD, that it can start early in life and affect young individuals, and that there are precursor conditions (“Pre-COPD”, “PRISm”), opens new windows of opportunity for its prevention, early diagnosis, and prompt and appropriate therapeutic intervention[72]. Importantly, several pharmacological and non-pharmacological therapies have now been shown to reduce mortality of COPD patients[Figure 6] but, in order to implement them, COPD must be diagnosed. Thus, any strategy aimed at addressing and improving the huge underdiagnosis of COPD in the community should be reinforced.
Acknowledgments
Authors acknowledge the support of Katie Langefeld and Ruth Hadfield for their careful editing of this 2023 GOLD report, as well as that of the members of the GOLD Emeriti Academy, GOLD Assembly and industry stakeholders for their comments and feed-back.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023. Available from: https://goldcopd.org/2023-gold-report-2/
- [Google Scholar]
- Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. American Journal of Respiratory and Critical Care Medicine. 2022;206:1317-1325.
- [CrossRef] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. 2022. Available from: www.goldcopd.org
- [Google Scholar]
- Pathogenesis of chronic obstructive pulmonary disease: understanding the contributions of gene-environment interactions across the lifespan. The Lancet Respiratory Medicine 2022. ;. ;10:512-524.
- [CrossRef] [PubMed] [Google Scholar]
- The Natural History of Chronic Airflow Obstruction Revisited: An Analysis of the Framingham Offspring Cohort. Am J Respir Crit Care Med. 2009;180:3-10.
- [CrossRef] [PubMed] [Google Scholar]
- COPD: the dangerous underestimate of 15%. Lancet. 2006;367:1216-1219.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of water-pipe smoking on lung function: a systematic review and meta-analysis. Chest. 2011;139:764-774.
- [CrossRef] [PubMed] [Google Scholar]
- Waterpipe tobacco smoking. Tuberkuloz ve toraks. 2016;64:94-96.
- [CrossRef] [PubMed] [Google Scholar]
- Chinese water-pipe smoking and the risk of COPD. Chest. 2014;146:924-931.
- [CrossRef] [PubMed] [Google Scholar]
- Marijuana and chronic obstructive lung disease: a population-based study. Cmaj. 2009;180:814-820.
- [CrossRef] [PubMed] [Google Scholar]
- Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet. 2007;370:751-757.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med. 1995;152:977-983.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. The Lancet Respiratory Medicine 2022
- [CrossRef] [PubMed] [Google Scholar]
- Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:542-546.
- [CrossRef] [PubMed] [Google Scholar]
- Household air pollution and COPD: cause and effect or confounding by other aspects of poverty? Int J Tuberc Lung Dis. 2022;26:206-216.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic obstructive pulmonary disease associated with biomass fuel use in women: a systematic review and meta-analysis. BMJ Open Respir Res. 2018;5:e000246.
- [CrossRef] [PubMed] [Google Scholar]
- The “Slow Horse Racing Effect” on Lung Function in Adult Life in Chronic Obstructive Pulmonary Disease Associated to Biomass Exposure. Front Med (Lausanne). 2021;8:700836.
- [CrossRef] [PubMed] [Google Scholar]
- Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:557-565.
- [CrossRef] [PubMed] [Google Scholar]
- The occupations at increased risk of COPD: analysis of lifetime job-histories in the population-based UK Biobank Cohort. European Respiratory Journal. 2019;54:1900186.
- [CrossRef] [PubMed] [Google Scholar]
- Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156:738-746.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health. 2018;2:e114-e125.
- [CrossRef] [PubMed] [Google Scholar]
- Ambient Air Pollution and Dysanapsis: Associations with Lung Function and Chronic Obstructive Pulmonary Disease in the Canadian Cohort Obstructive Lung Disease Study. American Journal of Respiratory and Critical Care Medicine. 2022;206:44-55.
- [CrossRef] [PubMed] [Google Scholar]
- Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3079-3091.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of α[1]-antitrypsin PiZZ genotypes in patients with COPD in Europe: a systematic review. Eur Respir Rev 2020:29.
- [CrossRef] [PubMed] [Google Scholar]
- Alpha-1 Antitrypsin Deficiency: The Learning Goes On. Am J Respir Crit Care Med. 2020;202:6-7.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics of chronic obstructive pulmonary disease: understanding the pathobiology and heterogeneity of a complex disorder. The Lancet Respiratory Medicine 2022
- [CrossRef] [PubMed] [Google Scholar]
- Lung function trajectories in health and disease. Lancet Respir Med. 2019;7:358-364.
- [CrossRef] [PubMed] [Google Scholar]
- Lung function in early adulthood and health in later life: a transgenerational cohort analysis. The Lancet Respiratory Medicine 2017. ;. ;5:935-945.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between supernormal lung function and long-term risk of hospitalisations and mortality: a population-based cohort study. European Respiratory Journal 2021:2004055.
- [CrossRef] [PubMed] [Google Scholar]
- Supernormal lung function and risk of COPD: A contemporary population-based cohort study. EClinicalMedicine. 2021;37:100974.
- [CrossRef] [PubMed] [Google Scholar]
- Association of birth weight with adult lung function: findings from the British Women's Heart and Health Study and a meta-analysis. Thorax. 2005;60:851-858.
- [CrossRef] [PubMed] [Google Scholar]
- Variability of maximum expiratory flow-volume curves. J Appl Physiol. 1974;37:67-74.
- [CrossRef] [PubMed] [Google Scholar]
- COPD as a disease of accelerated lung aging. Chest. 2009;135:173-180.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between maximal expiratory flows and lung volumes in growing humans. J Appl Physiol (1985). 1988;65:822-828.
- [CrossRef] [PubMed] [Google Scholar]
- The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525-534.
- [CrossRef] [PubMed] [Google Scholar]
- Association of Dysanapsis With Chronic Obstructive Pulmonary Disease Among Older Adults. JAMA. 2020;323:2268-2280.
- [CrossRef] [PubMed] [Google Scholar]
- Lifetime spirometry patterns of obstruction and restriction, and their risk factors and outcomes: a prospective cohort study. The Lancet Respiratory Medicine 2022
- [CrossRef] [Google Scholar]
- Lifetime lung function trajectories and COPD: when the train derails. The Lancet Respiratory medicine 2022
- [CrossRef] [PubMed] [Google Scholar]
- Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758-764.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med. 2015;175:1539-1549.
- [CrossRef] [PubMed] [Google Scholar]
- Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. New England Journal of Medicine. 2015;373:111-122.
- [CrossRef] [PubMed] [Google Scholar]
- Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012-2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597-611.
- [CrossRef] [PubMed] [Google Scholar]
- Women manifest more severe COPD symptoms across the life course. Int J Chron Obstruct Pulmon Dis. 2018;13:3021-3029.
- [CrossRef] [PubMed] [Google Scholar]
- The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur Respir J. 2017;49
- [CrossRef] [PubMed] [Google Scholar]
- Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991-996.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183:891-897.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. New England Journal of Medicine. 2016;374:1842-1852.
- [CrossRef] [PubMed] [Google Scholar]
- Combined Impact of Smoking and Early Life Exposures on Adult Lung Function Trajectories. Am J Respir Crit Care Med 2017
- [CrossRef] [Google Scholar]
- Chronic Bronchial Infection Is Associated with More Rapid Lung Function Decline in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2022;19:1842-1847.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Transl Med. 2021;9:390.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health. 2018;6:e193-e202.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic Instability and Reversals of Chronic Obstructive Pulmonary Disease Diagnosis in Individuals with Mild to Moderate Airflow Obstruction. Am J Respir Crit Care Med. 2017;196:306-314.
- [CrossRef] [PubMed] [Google Scholar]
- Should the diagnosis of COPD be based on a single spirometry test? NPJ Prim Care Respir Med. 2016;26:16059.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
- [CrossRef] [PubMed] [Google Scholar]
- "GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study" Respir Res. 2012;13:13.
- [CrossRef] [PubMed] [Google Scholar]
- Discriminative Accuracy of FEV1:FVC Thresholds for COPD-Related Hospitalization and Mortality. Jama. 2019;321:2438-2447.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67:701-708.
- [CrossRef] [PubMed] [Google Scholar]
- Counterpoint: Is an increase in FEV[1] and/or FVC >/= 12% of control and >/= 200 mL the best way to assess positive bronchodilator response? No. Chest. 2014;146:538-541.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2019;381:1248-1256.
- [CrossRef] [PubMed] [Google Scholar]
- Tiotropium in Early-Stage Chronic Obstructive Pulmonary Disease. N Engl J Med. 2017;377:923-935.
- [CrossRef] [PubMed] [Google Scholar]
- Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27:525-528.
- [CrossRef] [PubMed] [Google Scholar]
- At the Root: Defining and Halting Progression of Early Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018;197:1540-1551.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of Early COPD In Young Adults for Development of Clinical COPD: Findings from the Copenhagen General Population Study. Am J Respir Crit Care Med 2020
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic characterisation of early COPD: a prospective case-control study. ERJ Open Research. 2020;6:047-02020.
- [CrossRef] [PubMed] [Google Scholar]
- Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. European Respiratory Journal. 2014;44:324-331.
- [CrossRef] [PubMed] [Google Scholar]
- From GOLD 0 to Pre-COPD. American journal of respiratory and critical care medicine. 2021;203:414-423.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchodilators in Tobacco-Exposed Persons with Symptoms and Preserved Lung Function. The New England journal of medicine. 2022;387:1173-1184.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment Trials in Pre-COPD and Young COPD: Time to Move Forward. American journal of respiratory and critical care medicine 2021 in press
- [Google Scholar]
- Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89.
- [CrossRef] [PubMed] [Google Scholar]
- The Clinical Spectrum of PRISm. American journal of respiratory and critical care medicine. 2022;206:524-525.
- [CrossRef] [PubMed] [Google Scholar]
- Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400:921-972.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155-161.
- [CrossRef] [PubMed] [Google Scholar]
- GOLD 20th anniversary: a brief history of time. Eur Respir J. 2017;50:1700671.
- [CrossRef] [PubMed] [Google Scholar]
- Different dyspnoea perception in COPD patients with frequent and infrequent exacerbations. Thorax. 2017;72:117-121.
- [CrossRef] [PubMed] [Google Scholar]
- CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635-640.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchial Infection and Temporal Evolution of Bronchiectasis in Patients With Chronic Obstructive Pulmonary Disease. Clin Infect Dis. 2021;72:403-410.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. New England Journal of Medicine. 2011;365:395-409.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. New England Journal of Medicine 2020
- [CrossRef] [PubMed] [Google Scholar]
- Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nature medicine. 2012;18:1711-1715.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive Imaging Biomarker Identifies Small Airway Damage in Severe Chronic Obstructive Pulmonary Disease. American journal of respiratory and critical care medicine. 2019;200:575-581.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2016;194:178-184.
- [CrossRef] [PubMed] [Google Scholar]
- Chest CT-assessed comorbidities and all-cause mortality risk in COPD patients in the BODE cohort. Respirology. 2022;27:286-293.
- [CrossRef] [PubMed] [Google Scholar]
- A Systematic Review of Published Algorithms for Selecting an Inhaled Delivery System in Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society. 2022;19:1213-1220.
- [CrossRef] [PubMed] [Google Scholar]
- Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J. 2014;43:1599-1609.
- [CrossRef] [PubMed] [Google Scholar]
- Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109:1312-1319.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respiratory Research. 2019;20:238.
- [CrossRef] [PubMed] [Google Scholar]
- Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. New England Journal of Medicine. 2018;378:1671-1680.
- [CrossRef] [PubMed] [Google Scholar]
- Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. New England Journal of Medicine. 2020;383:35-48.
- [CrossRef] [PubMed] [Google Scholar]
- Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. American Journal of Respiratory and Critical Care Medicine. 2022;205:17-35.
- [CrossRef] [PubMed] [Google Scholar]
- Treatable Traits: Toward Precision Medicine of Airway Diseases. Eur Respir J. 2016;47:410-419.
- [CrossRef] [PubMed] [Google Scholar]
- Treatable traits in the NOVELTY study. Respirology. 2022;27:929-940.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Roflumilast and Inhaled Corticosteroid/Long-Acting β2-Agonist on Chronic Obstructive Pulmonary Disease Exacerbations (RE2SPOND) A Randomized Clinical Trial. American Journal of Respiratory and Critical Care Medicine. 2016;194:559-567.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur Respir J. 2017;50
- [CrossRef] [PubMed] [Google Scholar]
- Azithromycin for Prevention of Exacerbations of COPD. New England Journal of Medicine. 2011;365:689-698.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of COPD Exacerbation Reduction in Response to Daily Azithromycin Therapy. American Journal of Respiratory and Critical Care Medicine 2014
- [CrossRef] [PubMed] [Google Scholar]
- Withdrawal of Inhaled Glucocorticoids and Exacerbations of COPD. New England Journal of Medicine. 2014;371:1285-1294.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2015;192:523-525.
- [CrossRef] [PubMed] [Google Scholar]
- Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. The Lancet Respiratory Medicine 2015
- [CrossRef] [PubMed] [Google Scholar]
- Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. The Lancet 2017
- [CrossRef] [PubMed] [Google Scholar]
- Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled corticosteroids in COPD: Friend or foe? Eur Respir J. 2018;52:1801219.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Eosinophils and Chronic Obstructive Pulmonary Disease: A Global Initiative for Chronic Obstructive Lung Disease Science Committee 2022 Review. American Journal of Respiratory and Critical Care Medicine. 2022;206:17-24.
- [CrossRef] [PubMed] [Google Scholar]
- Stability of Blood Eosinophil Count in Patients with COPD in the UK Clinical Practice Research Datalink. Copd. 2017;14:382-388.
- [CrossRef] [PubMed] [Google Scholar]
- Stability of Blood Eosinophils in COPD and Controls and the Impact of Gender, Age, Smoking and Baseline Counts. American Journal of Respiratory and Critical Care Medicine. ;0 null
- [Google Scholar]
- The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: a systematic review. Lancet Glob Health. 2022;10:e1423-e1442.
- [CrossRef] [PubMed] [Google Scholar]
- Smoking Cessation/Vaccinations. Clin Chest Med. 2020;41:495-512.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972-977.
- [CrossRef] [PubMed] [Google Scholar]
- Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48:69-81.
- [CrossRef] [PubMed] [Google Scholar]
- An official European Respiratory Society statement on physical activity in COPD. European Respiratory Journal. 2014;44:1521-1537.
- [CrossRef] [PubMed] [Google Scholar]
- Using a smartphone application maintains physical activity following pulmonary rehabilitation in patients with COPD: a randomised controlled trial. Thorax 2022
- [CrossRef] [PubMed] [Google Scholar]
- An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. American Journal of Respiratory and Critical Care Medicine. 2013;188:e13-e64.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J. 2016;47:1336-1341.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Pulmonary Disease: Review of Selected Guidelines: an official statement from the American Association of cardiovascular and pulmonary rehabilitation. Journal of cardiopulmonary rehabilitation and prevention. 2016;36:75-83.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of Referral to Pulmonary Rehabilitation from UK Primary Care. Int J Chron Obstruct Pulmon Dis. 2020;15:2941-2952.
- [CrossRef] [PubMed] [Google Scholar]
- Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;1:Cd013040.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary rehabilitation at a time of social distancing: prime time for tele-rehabilitation? Thorax. 2020;75:446-447.
- [CrossRef] [PubMed] [Google Scholar]
- Home-based or remote exercise testing in chronic respiratory disease, during the COVID-19 pandemic and beyond: A rapid review. Chron Respir Dis. 2020;17:1479973120952418.
- [CrossRef] [PubMed] [Google Scholar]
- An Updated Definition and Severity Classification of COPD Exacerbations: The Rome Proposal. American Journal of Respiratory and Critical Care Medicine. 2021;204:1251-1258.
- [CrossRef] [PubMed] [Google Scholar]
- Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J. 2013;41:993-995.
- [CrossRef] [PubMed] [Google Scholar]
- Exacerbations in COPD: a personalized approach to care. Lancet Respir Med 2023 (in press)
- [CrossRef] [PubMed] [Google Scholar]
- Oxygen versus air-driven nebulisers for exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. BMC Pulm Med. 2018;18:157.
- [CrossRef] [PubMed] [Google Scholar]
- Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta-analysis of randomised trials. Bmj. 2003;327:643.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous aminophylline in patients admitted to hospital with non-acidotic exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Thorax. 2005;60:713-717.
- [CrossRef] [PubMed] [Google Scholar]
- Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354:456-460.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2002;165:698-703.
- [CrossRef] [PubMed] [Google Scholar]
- Outpatient Oral Prednisone after Emergency Treatment of Chronic Obstructive Pulmonary Disease. The New England Journal of Medicine. 2003;348:2618.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: The reduce randomized clinical trial. JAMA. 2013;309:2223-2231.
- [CrossRef] [PubMed] [Google Scholar]
- COPD exacerbations: the impact of long versus short courses of oral corticosteroids on mortality and pneumonia: nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Respir Res. 2019;6:e000407.
- [CrossRef] [PubMed] [Google Scholar]
- Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest. 2007;132:1741-1747.
- [CrossRef] [PubMed] [Google Scholar]
- The role of nebulised budesonide in the treatment of exacerbations of COPD. Eur Respir J. 2007;29:660-667.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Eosinophils to Direct Corticosteroid Treatment of Exacerbations of Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2012;186:48-55.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- [CrossRef] [PubMed] [Google Scholar]
- Are short courses of antibiotic therapy as effective as standard courses for COPD exacerbations? A systematic review and meta-analysis. Pulm Pharmacol Ther. 2022;72:102111.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Pulmonary Embolism Among Patients With COPD Hospitalized With Acutely Worsening Respiratory Symptoms. JAMA. 2021;325:59-68.
- [CrossRef] [Google Scholar]
- Effect of a Pulmonary Embolism Diagnostic Strategy on Clinical Outcomes in Patients Hospitalized for COPD Exacerbation: A Randomized Clinical Trial. JAMA. 2021;326:1277-1285.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462.
- [CrossRef] [PubMed] [Google Scholar]
- Oximetry neither to prescribe long-term oxygen therapy nor to screen for severe hypoxaemia. ERJ Open Research. 2021;7:272-02021.
- [CrossRef] [PubMed] [Google Scholar]
- Racial Bias in Pulse Oximetry Measurement. N Engl J Med. 2020;383:2477-2478.
- [CrossRef] [PubMed] [Google Scholar]
- Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20:109.
- [CrossRef] [PubMed] [Google Scholar]
- Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71:759-761.
- [CrossRef] [PubMed] [Google Scholar]
- Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am J Respir Crit Care Med. 2017;195:1207-1215.
- [CrossRef] [PubMed] [Google Scholar]
- Home High-Flow Nasal Cannula Oxygen Therapy for Stable Hypercapnic COPD: A Randomized Clinical Trial. Am J Respir Crit Care Med. 2022;206:1326-1335.
- [CrossRef] [PubMed] [Google Scholar]
- High-flow nasal cannula versus conventional oxygen therapy in acute COPD exacerbation with mild hypercapnia: a multicenter randomized controlled trial. Crit Care. 2022;26:109.
- [CrossRef] [PubMed] [Google Scholar]
- ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022;59
- [CrossRef] [PubMed] [Google Scholar]
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation--a consensus conference report. Chest. 1999;116:521-534.
- [CrossRef] [PubMed] [Google Scholar]
- Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:Cd004104.
- [CrossRef] [PubMed] [Google Scholar]
- Discontinuing noninvasive ventilation in severe chronic obstructive pulmonary disease exacerbations: a randomised controlled trial. Eur Respir J. 2017;50
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185:152-159.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for all-cause hospital readmission following exacerbation of COPD: a systematic review and meta-analysis. Eur Respir Rev. 2020;29
- [CrossRef] [PubMed] [Google Scholar]
- Health Coaching and Chronic Obstructive Pulmonary Disease Rehospitalization. A Randomized Study. Am J Respir Crit Care Med. 2016;194:672-680.
- [CrossRef] [PubMed] [Google Scholar]
- The Association Between Hospital Readmission and Pulmonologist Follow-up Visits in Patients With COPD. Chest. 2015;148:375-381.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive properties of different multidimensional staging systems in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:521-526.
- [CrossRef] [PubMed] [Google Scholar]
- Reassessment of Home Oxygen Prescription after Hospitalization for Chronic Obstructive Pulmonary Disease. A Potential Target for Deimplementation. Ann Am Thorac Soc. 2021;18:426-432.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011:Cd005305.
- [CrossRef] [Google Scholar]
- Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12:Cd005305.
- [CrossRef] [PubMed] [Google Scholar]
- Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37:508-515.
- [CrossRef] [PubMed] [Google Scholar]
- Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine (Baltimore). 2016;95:e4225.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10:81-89.
- [CrossRef] [PubMed] [Google Scholar]
- High-risk patients following hospitalisation for an acute exacerbation of COPD. Eur Respir J. 2013;42:946-955.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic respiratory abnormalities in the multi-morbid frail elderly. BRN Reviews 2017 (in press)
- [CrossRef] [Google Scholar]
- Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962-969.
- [CrossRef] [PubMed] [Google Scholar]
- Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease: Incidence and Predicting Factors. American Journal of Respiratory and Critical Care Medicine. 2011;184:913-919.
- [CrossRef] [PubMed] [Google Scholar]
- Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the “Chronic Obstructive Pulmonary Disease-Bronchiectasis Association”. American Journal of Respiratory and Critical Care Medicine. 2022;206:417-426.
- [CrossRef] [PubMed] [Google Scholar]
- High Prevalence of Obstructive Sleep Apnea in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2015;12:1219-1225.
- [CrossRef] [Google Scholar]
- The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med. 2007;101:177-185.
- [CrossRef] [PubMed] [Google Scholar]
- The Prevalence of Metabolic Syndrome In Chronic Obstructive Pulmonary Disease: A Systematic Review. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2016;13:399-406.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine. 2010;363:1128-1138.
- [CrossRef] [PubMed] [Google Scholar]
- Gastro-esophageal reflux disease and exacerbations in chronic obstructive pulmonary disease. Respirology. 2015;20:101-107.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc. 2009;57:1453-1457.
- [CrossRef] [PubMed] [Google Scholar]
- Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology. 2016;21:883-890.
- [CrossRef] [PubMed] [Google Scholar]
- Anemia and Adverse Outcomes in a Chronic Obstructive Pulmonary Disease Population with a High Burden of Comorbidities. An Analysis from SPIROMICS. Annals of the American Thoracic Society. 2018;15:710-717.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of hemoglobin on pulmonary arterial pressure and pulmonary vascular resistance in patients with chronic emphysema. Respiration. 2000;67:502-506.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and predictors associated with severe pulmonary hypertension in COPD. The American journal of emergency medicine. 2018;36:277-280.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199-208.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005;128:1201-1208.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. American journal of respiratory and critical care medicine. 2011;183:604-611.
- [CrossRef] [PubMed] [Google Scholar]
- Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127:1205-1211.
- [CrossRef] [PubMed] [Google Scholar]
- Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60-67.
- [CrossRef] [PubMed] [Google Scholar]
- Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134:43S-56S.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of anxiety on health outcomes in COPD. Thorax. 2010;65:229-234.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:631-639.
- [CrossRef] [PubMed] [Google Scholar]
- Domain-specific cognitive impairment in patients with COPD and control subjects. Int J Chron Obstruct Pulmon Dis. 2017;12:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive impairment and clinical characteristics in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2018;15:91-102.
- [CrossRef] [PubMed] [Google Scholar]
- Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-156.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis. Bmj. 2021;375:e068302.
- [CrossRef] [PubMed] [Google Scholar]
- Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The 2020 GOLD Science Committee Report on COVID-19 & COPD. American Journal of Respiratory and Critical Care Medicine. 2021;203:24-36.
- [CrossRef] [PubMed] [Google Scholar]
- Reduction in hospitalised COPD exacerbations during COVID-19: A systematic review and meta-analysis. PLoS One. 2021;16:e0255659.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, Healthcare Resource Utilization, and Mortality of Asthma and COPD in COVID-19: A Systematic Literature Review and Meta-Analyses. J Asthma Allergy. 2022;15:811-825.
- [CrossRef] [PubMed] [Google Scholar]
- Reduction in All-Cause Mortality with Fluticasone Furoate/Umeclidinium/Vilanterol in COPD Patients. Am J Respir Crit Care Med. 2020;201:1508-1516.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239.
- [CrossRef] [PubMed] [Google Scholar]
- Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med. 2018;18:154.
- [CrossRef] [PubMed] [Google Scholar]
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. A clinical trial. Ann Intern Med. 1980;93:391-398.
- [CrossRef] [PubMed] [Google Scholar]
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;i:681-685.
- [CrossRef] [Google Scholar]
- Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2:698-705.
- [CrossRef] [PubMed] [Google Scholar]
- A Randomized Trial Comparing Lung-Volume-Reduction Surgery with Medical Therapy for Severe Emphysema. The New England Journal of Medicine. 2003;348:2059-2073.
- [CrossRef] [PubMed] [Google Scholar]





