Translate this page into:
Comparative effectiveness of shorter regimen with oral bedaquiline or injectable on treatment outcomes and mortality among patients with multidrug-resistant tuberculosis in Guinea: A retrospective cohort study
*Corresponding author: Boubacar Djelo Diallo, Faculté des Sciences et Techniques de la Santé, Université Gamal Abdel Nasser de Conakry, Service de Pneumo-Phtisiologie, CHU Conakry, Hôpital National Ignace Deen de Conakry. diallodjelo@yahoo.fr
-
Received: ,
Accepted: ,
How to cite this article: Diallo B, Diallo A, Diallo O, Barry A, Magassouba A, Camara L. Comparative effectiveness of shorter regimen with oral bedaquiline or injectable on treatment outcomes and mortality among patients with multidrug-resistant tuberculosis in Guinea: A retrospective cohort study. J Pan Afr Thorac Soc. 2024;5:122-6. doi: 10.25259/JPATS_2_2024
Abstract
Objectives:
Results of clinical trials indicate that oral bedaquiline, instead of an injectable drug to treat rifampicin-resistant tuberculosis (RR-TB), is associated with significant improvement in treatment success and mortality 24 months after treatment initiation. We aimed to compare treatment success and mortality in patients treated for multidrug-resistant tuberculosis (MDR-TB) with shorter oral bedaquiline-containing versus injectable regimens in Guinea.
Materials and Methods:
We enrolled patients with RR-TB who were treated with an MDR-TB treatment regimen from June 2016 to June 2022 in three tuberculosis (TB) centers in Guinea. The primary outcome was mortality, and the secondary outcomes were treatment success and loss of follow-up. A based on average treatment effect on the treated propensity score on age, sex, geographic site, previous TB treatment, acid-fast-bacilli smear-positivity, and human immunodeficiency virus-infection status was used to account for confounding bias. Cox and logistic regression models were used to obtain adjusted hazard ratios (HR) and odds ratios (OR).
Results:
1112 patients treated for MDR-TB during the study period were analyzed: 253 in the bedaquiline group and 859 in the injectable group. Fifteen patients (5.9%) were lost to follow-up, 18 (6.7%) had treatment failure or recurrence, and 46 died (18.2%). A 175 (69.2%) had treatment success in the bedaquiline group, compared with 57 (6.6%), 21 (2.4%), 162 (18.9%), and 619 (72.1%) in the injectable group, respectively. In the adjusted analyses, the bedaquiline-containing regimen was associated with a significant reduction of all-cause mortality (HR: 0.62, 95% confidence interval CI]: 0.42–0.91), a higher probability of treatment success (OR: 1.08, 95% CI: 1.00–1.17), and a similar risk of loss to follow-up (HR: 0.62, 95% CI: 0.32–1.22) as compared with the injectable group.
Conclusion:
In real-life conditions, a short oral bedaquiline regimen was associated with lower odds of death and higher odds of treatment success among patients with MDR-TB. These findings support the use of short bedaquiline-containing regimens until a new BPaLM regimen is widely available.
Keywords
Bedaquiline
Multidrug-resistant
Tuberculosis
INTRODUCTION
Tuberculosis (TB) is the foremost infectious disease globally, and almost a billion people have died due to TB over the past two centuries.[1] In 2022, TB was the world’s second leading cause of death from a single infectious agent after COVID-19 and caused almost twice as many deaths as human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome.[2] More than 10 million people continue to fall ill with TB every year,[2] and around half a million cases of multidrug-resistant TB (MDR-TB) are diagnosed worldwide.[3] Despite numerous efforts to improve the management of these patients, cure rates remain below the thresholds set by the World Health Organization (WHO).[2] This rate is close to 70% in Guinea, while the incidence of MDR-TB continues to rise.[4] Under the impetus of the WHO, health authorities are modifying national guidelines as new data emerges. In 2022, the WHO recommended the use of the 6 months all-oral treatment regimen composed of bedaquiline, pretonamid, linezolid (600 mg), and moxifloxacin (BPaLM) rather than 9 months or longer (18 months) regimens in MDR/rifampicin (RIF)-resistant tuberculosis patients.[5] However, the availability and implementation of this new strategy could take time, depending on the responsiveness of healthcare systems in many countries. This is one of the reasons why the classic oral regimen, including bedaquiline, continues to be used in many developing countries. The real-life efficacy of this short all-oral regimen containing bedaquiline compared with a short injectable regimen containing kanamycin or amikacin was demonstrated in a large cohort of patients in South Africa.[6,7] In Guinea, this short oral regimen, including bedaquiline, was introduced in 2021.[8] In the present study, we aimed to compare the shorter oral bedaquiline-containing versus injectable regimens on treatment success and mortality in patients treated for MDR-TB in Guinea.
MATERIALS AND METHODS
Study design and population
We conducted a retrospective, multicenter, longitudinal, and cohort study at three referral drug-resistance TB centers in Guinea (University Hospital of Ignace-Deen, Carrière Center, and Tombolia Center). The Wanindara and Carrière are first-level health facilities providing outpatient care for MDR-TB patients. The pneumology department is a university hospital department with 75 inpatient beds.
We analyzed patients with RIF resistance who received an MDR-TB treatment regimen between June 07, 2016, and June 22, 2022. In Guinea, as recommended by the WHO, any patient with RIF resistance diagnosed with the Xpert Mycobacterium tuberculosis/RIF was treated as MDR-TB.
MDR-TB regimens
According to the guideline for MDRTB management in Guinea, patients naive to second-line anti-TB drugs were treated with a 9–12 months regimen consisting of an intensive phase lasting a minimum of 4–6 months followed by 5–6 months of a continuation phase. We classified patients into two groups according to their treatment regimen: (1) Injectable group: The short WHO-recommended injectable regimen including moxifloxacin, kanamycin or amikacin, clofazimine, protionamide, pyrazinamide, ethambutol, and isoniazid at high dose, and (2) bedaquiline group: the short, all-oral bedaquiline regimen where injectable drugs were replaced by bedaquiline. Patients were seen at baseline and followed up at monthly visits for 9–12 months.
Outcome and predictive variables
According to the 2013 revised WHO recommendations, we classified TB treatment outcomes into two categories (successful or unsuccessful).[9] Successful treatment outcomes corresponded to patients who were declared as either “cured” or “treatment completed.” Unsuccessful treatment outcomes included “treatment failure,” “death for any reason,” “lost to follow-up,” or “not evaluated.” Demographic and baseline clinical data included center, age, gender, residence, HIV status, previous TB treatment with first-line drugs, and results of smear and culture. Information was collected using a case report form from the national MDR-TB registry initiated in 2016.
Statistical analysis
Descriptive statistics (frequencies and percentages or mean and standard deviation [SD]) were used to describe the demographic and clinical characteristics of participants at baseline. The primary outcome was all-cause mortality, and secondary outcomes were treatment success and loss of follow-up. To account for confounding bias, we computed a propensity score using a caliper distance of 0.025 SD on age, sex, geographic site, previous TB treatment, acid-fast-bacilli smear positivity, and HIV infection status [Appendix Figures 1 and 2]. The standardized mean difference method was used to assess the balance of covariates between the vaccination groups before and after weighting. Adjusted Cox and logistic regressions on propensity score were applied to estimate the average effect on TB regimen as the inverse probability of treatment weighting using the average treatment effect on the treated method and to obtain adjusted hazard ratios (HR) and odds ratios (OR). Subgroup analyses were performed according to HIV status and gender. All data analyses were done in R (version 3.5.1). Significance was defined as P < 0.05, and all tests were two-sided.
RESULTS
A total of 1193 patients were retrieved from the national MDR-TB registry in June 2022. Among these patients, we excluded 81 (6.8%) who had previously received treatment with second-line drugs, making them ineligible for short regimens and 64 (5.3%) who had missing value outcomes. Of the remaining 1048 patients who were analyzed, 225 (21.5%) were in the bedaquiline group and 823 (78.5%) in the injectable group [Figure 1].
- Study population selection.
Patients in the bedaquiline group were older, with a mean age of 36.3 years (SD: 14.0) versus 33.1 years (SD: 12.5; P = 0.002) in the injectable group. Patients in the injectable group were more likely to have a 3+ positive smear (32.2% vs. 28.9%; P < 0.001). There were similar proportions of women and HIV status [Table 1].
| Bedaquiline group (n=225) | Injectable group (n=823) | P-value | |
|---|---|---|---|
| Patients characteristics | |||
| Mean (SD) age | 36.3 (14.0) | 33.1 (12.5) | 0.002 |
| Sex (%) | |||
| Male | 154 (68.4) | 576 (70.0) | 0.716 |
| Female | 71 (31.6) | 247 (30.0) | |
| HIV infection (%) | 50 (22.2) | 167 (20.3) | 0.589 |
| AFB smear positive (%) | 147 (65.3) | 603 (79.3) | <0.001 |
| TB culture positive (%) | 120 (53.3) | 393 (47.8) | 0.028 |
| Previously treated for drug susceptible TB (%) | 24 (10.7) | 17 (2.07) | <0.001 |
| Treatment site (%) | |||
| CHU Ignace Deen | 51 (22.7) | 215 (26.1) | 0.101 |
| Carrière | 71 (31.6) | 296 (36.0) | |
| Tombolia | 103 (45.8) | 312 (37.9) | |
| End of TB treatment outcomes (%) | |||
| Treatment success | 161 (71.6) | 602 (73.1) | |
| Failure | 12 (5.33) | 20 (2.43) | |
| Recurrence | 4 (1.78) | 0 (0.00) | |
| Death | 35 (15.6) | 146 (17.7) | |
| Lost to follow-up | 13 (5.78) | 55 (6.68) |
Data are expressed as n=number (%) for categorical variable and mean (SD); SD=Standard deviation for continuous variable. TB: Tuberculosis, HIV: Human immunodeficiency virus, AFB: Acid-fast bacillus, CHU: University Hospital Center, Bold: Significant at the 5% threshold
At the end of treatment, 161 (71.6%) patients in the bedaquiline group and 602 (73.1%) in the injectable group were considered as treatment success. Among patients included in the bedaquiline group, 35 (15.6%) died, 16 (7.1%) had treatment failure or recurrence, and 13 (5.8%) were lost to follow-up. The corresponding end-treatment outcomes in patients included in the injectable group were 146 (17.7%), 20 (2.4%), and 55 (6.7%), respectively. In the adjusted analyses, the bedaquiline-containing regimen was associated with a reduction of all-cause mortality by 38% (HR = 0.62, 95% confidence interval [CI], 0.42–0.91), with a higher probability of treatment success (OR = 1.08, 1.00–1.17) and a similar risk of loss to follow-up (HR = 0.62, 0.32–1.22), compared with the injectable group. In subgroup analyses, we found that women included in the bedaquiline group had a 56% reduction in mortality (HR = 0.46, 0.24–0.89) and a higher probability of treatment success (OR = 1.15, 1.00–1.32) compared with women in the injectable group [Figure 2].
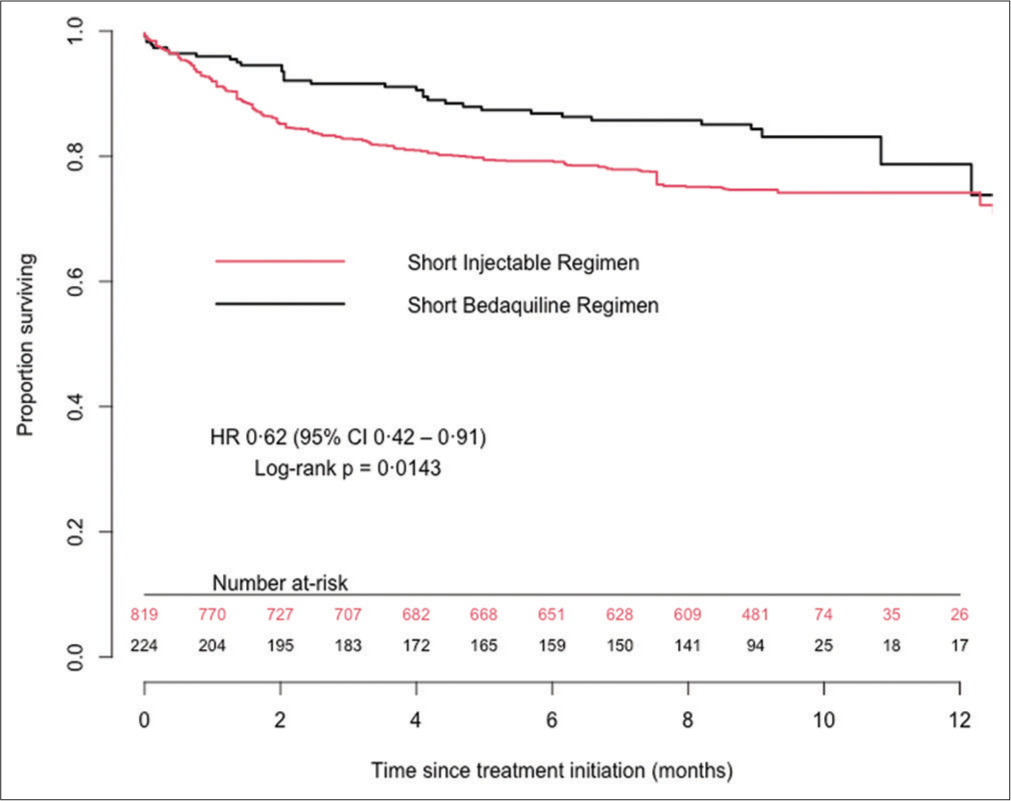
- Kaplan–Meier survival curves up to 12 months after TB treatment initiation. CI: Confidence interval. HR: Hazard ratio, TB: Tuberculosis.
A similar effect between bedaquiline and the injectable groups in terms of reducing mortality and the number of patients lost to follow-up, and an increased probability of successful treatment was observed for men regardless of HIV status [Table 2].
| End of TB treatment outcomes | HR | 95% CI | P-value |
|---|---|---|---|
| Total population | |||
| Treatment success* | 1.08 | 1.00–1.17 | 0.044 |
| Death | 0.62 | 0.42–0.91 | 0.014 |
| Lost to follow-up | 0.62 | 0.32–1.22 | 0.166 |
| Women | |||
| Treatment success* | 1.15 | 1.00–1.32 | 0.047 |
| Death | 0.46 | 0.24–0.89 | 0.021 |
| Lost to follow-up | 0.44 | 0.08–2.48 | 0.350 |
| Men | |||
| Treatment success* | 1.05 | 0.96–1.15 | 0.288 |
| Death | 0.73 | 0.46–1.17 | 0.195 |
| Lost to follow-up | 0.66 | 0.32–1.37 | 0.265 |
| HIV infection | |||
| Treatment success* | 1.02 | 0.86–1.20 | 0.831 |
| Death | 0.75 | 0.44–1.29 | 0.295 |
| Lost to follow-up | 1.11 | 0.24–5.11 | 0.897 |
HR: Hazard ratio, CI: Confidence interval, HIV: Human immunodeficiency virus, TB: Tuberculosis. Bold: Significant at the 5% threshold. *Estimation was an odds ratio from logistic regression.
DISCUSSION
In this large cohort of patients treated for MDR-TB in Guinea, we found that the bedaquiline-containing regimen was associated with lower risks of mortality and loss of follow-up and a higher probability of treatment success. Importantly, women treated with the bedaquiline-containing regimen had more benefits than those from the injectable group. Although women were younger and more frequently HIV-infected than men, this observed benefit appeared to be independent of HIV status. However, it should be pointed out that many authors reported more unfavorable treatment outcomes in cases of MDR-TB and HIV co-infection.[3,10,11] Depending on the year, the therapeutic success rate for MDR-TB in Guinea varies between 70% and 75%.[12,13] The loss to follow-up rate was almost identical in the two groups.
Our results are consistent with those reported in a South African cohort.[7] Nevertheless, in our study, the success rate in the bedaquiline arm was 71.6%, slightly lower than what was reported in the South African cohort (74%) and a large meta-analysis (74.7%).
Our study has a number of strengths. First, it analyzed a large sample of patients from three referral centers for MDR-TB management in Guinea, which reduced selection bias and increased the validity of the extrapolation of our findings to the entire population of Guinean patients with MDR-TB. Second, our results are consistent with those reported in a South African cohort. The statistical methods used to analyze the data were similar in both cohorts. These two cohorts were very comparable in terms of participant profiles and statistical methods used to analyze the data. However, the limits of our study were its retrospective design and small sample size, which had an impact on the internal validity of the study, and some missing information on antiretroviral treatment (ART) initiation, which could possibly serve as a proxy for MDRTB assessment. Further prospective cohort studies are needed to confirm our findings.
Our results are consistent with the work carried out in Africa, which justified the WHO recommendations on the use of bedaquiline in the treatment of MDR TB. Treatment regimens with bedaquiline have a better therapeutic outcome, are better tolerated, and easier to administer. Treatment regimens for MDR TB using injectable drugs are no longer justified in our context, regardless of the adverse effects, which were not analyzed in our study.
Even more effective and shorter antituberculosis treatments (BPal and BPal M) have been developed in recent years and have been recommended by the WHO.[14] These treatments can be used for both MDR TB and extensively drug-resistant TB and are already in use in many countries. Guinea, like other countries, has updated its national guidelines to switch to BPAL and BPAL M as MDR-TB treatment for all eligible patients. However, due to the high toxicity of these treatments, it is more than necessary to find treatments with better safety.
CONCLUSION
Pending the availability of the 6-month BPaLM regimen in Guinea, the bedaquiline-containing regimen was associated with a significant improvement in TB treatment outcomes and should replace the injectable-containing regimen in the management of MDR-TB.
Acknowledgments
We thank the National Tuberculosis Control Program for its collaboration and Action Damien for its patients’ support during their treatment. We thank Dr. Laila Mbouemboue of the Koffi Annan Medical Sciences University (Guinea Conakry) for her help in revising the English of the manuscript.
Authors’ contributions
BDD contributed to the conception of the study, organization of the research project, supervision of data collection, and drafted the manuscript, AD organization of the research project, conceived the study design, analyzed the data, and drafted the manuscript, AOB, collected data, OHD and LMC contributed to the conception of the study, organization of the research project, and interpreted the manuscript. All authors approved the final version of the manuscript.
Availability of data and materials
The data are available on request (diallodjelo@yahoo.fr).
Ethical approval
The study was approved by the National Ethics Committee for Health Research (NECHR) attached to the Ministry of Health (Conakry, Guinea). The study was conducted in accordance with the Declaration of Helsinki, and the confidentiality of the data was guaranteed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Pretomanid with bedaquiline and linezolid for drug-resistant TB: A comparison of prospective cohorts. Int J Tuberc Lung Dis. 2021;25:45360.
- [CrossRef] [PubMed] [Google Scholar]
- World Health Organization: Global tuberculosis report 2023 Geneva, Swizerland: WHO Press; 2023.
- [Google Scholar]
- Multidrug-resistant tuberculosis (MDR-TB) and multidrug-resistant HIV (MDR-HIV) syndemic: Challenges in resource limited setting. BMJ Case Rep. 2019;12:230628.
- [CrossRef] [PubMed] [Google Scholar]
- Different profiles of body mass index variation among patients with multidrug-resistant tuberculosis: A retrospective cohort study. BMC Infect Dis. 2020;20:315.
- [CrossRef] [PubMed] [Google Scholar]
- A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N Engl J Med. 2022;387:233143.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: A retrospective cohort study. Lancet Respir Med. 2018;6:699706.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: A retrospective cohort study. Lancet Infect Dis. 2022;22:104251.
- [CrossRef] [PubMed] [Google Scholar]
- National tuberculosis control program Guinea: Annual report of TB control activity 2023.
- [Google Scholar]
- WHO: Definitions and reporting framework for tuberculosis - 2013 revision (updated 2014) Geneva, Switzerland: World Health Organization; 2013.
- [Google Scholar]
- Management of MDR-TB in HIV co-infected patients in Eastern Europe: Results from the TB: HIV study. J Infect. 2018;76:4454.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of TB/HIV co-infection and patterns of multidrug-resistance tuberculosis in the Northwest Amhara, Ethiopia. Infect Drug Resist. 2023;16:382945.
- [CrossRef] [PubMed] [Google Scholar]
- Better programmatic outcome with the shorter regimen for the treatment of multidrug-resistant tuberculosis (MDR-TB) in Guinea: A retrospective cohort study. PLoS One. 2020;15:237355.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a prognosis nomogram of treatment outcomes for MDR-tuberculosis in Guinea (Conakry): A retrospective cohort analysis. Cent Afr J Public Health. 2020;6:33.
- [CrossRef] [Google Scholar]
- Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893902.
- [CrossRef] [PubMed] [Google Scholar]







