Translate this page into:
Bronchoscopic spigot occlusion of tuberculosis-associated bronchopleural fistula in a human immunodeficiency virus-positive high-risk patient
*Corresponding author: Naveed Mohamoud Merali, Department Pulmonology, Avenue Hospital, Parklands, Nairobi, Kenya. naveed.merali@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Merali NM, Balasubramanian V, Munyu PW, Gajjar NE, Patel K, Samia B, et al. Bronchoscopic spigot occlusion of tuberculosis-associated bronchopleural fistula in a human immunodeficiency virus-positive high-risk patient. J Pan Afr Thorac Soc. doi: 10.25259/JPATS_2_2025
Abstract
A bronchopleural fistula (BPF) is defined as an abnormal communication between an airway and the pleural space. This often can arise from complications after thoracic surgical interventions or from severe cavitating lung infections such as tuberculosis (TB). Management of BPF usually involves surgical repair through video-assisted thoracoscopy; however, this can be challenging in high-risk patients who are unsuitable for surgery. Bronchoscopic interventions are effective treatment options in patients who are unsuitable for surgery and involve the placement of valves, glues, coils, or spigots to seal the leaking bronchus. This case describes a successful BPF closure in a high-risk human immunodeficiency virus-positive patient with gastric malignancy who sustained a fistula as a result of cavitatory pulmonary TB. The procedure was done using the endobronchial Watanabe spigot, which is a cost-effective option for low-income countries. This procedure, done for the 1st time in Kenya, underscores advancements in Kenyan interventional pulmonology, offering promising outcomes in the management of BPF, in the developing interventional pulmonology sector in Kenya.
Keywords
Bronchopleural fistula
Bronchoscopy
Spigot
Tuberculosis
INTRODUCTION
A bronchopleural fistula (BPF) is an abnormal communication between the main stem, lobar, or segmental bronchus, and the pleural space.[1] This can occur as a complication post-thoracic surgical procedure or due to structural lung diseases such as chronic obstructive pulmonary disease, trauma, interstitial lung diseases, and infections such as tuberculosis (TB) and lung abscesses. The burden of infections such as TB is higher in developing countries such as Kenya, and complications can be both difficult and expensive to treat, especially when there is a paucity of skilled workers such as cardiothoracic surgeons or interventional pulmonologists.[2]
There is no clear consensus on the optimal management of these fistulas due to varied success with the different treatment approaches. Conventionally, as per the European Institute of Oncology algorithm, management initially involves chest tube drainage to facilitate evacuation, antibiotic cover, and supportive care.[3] Surgical closure can be undertaken in suitable patients and early cases through direct closure and by the use of a vascularized flap.[3-5] Surgical repair in unstable patients or those likely to have friable and inflamed lung tissue is generally deferred due to the high likelihood of failure. Patients with co-morbidities such as human immunodeficiency virus (HIV) coinfection or malignancy are also poorer surgical candidates and carry a higher surgical risk.[3,6]
In high-risk patients, bronchoscopic occlusion of the culprit bronchus is a valid therapeutic modality as it poses a lower surgical risk and good outcomes.[5,7] Methods used include cyanoacrylate glues, fibrin gels, and placement of occlusive devices such as Amplatzer devices, valves, coils, stents, or spigots such as the Watanabe spigot.
The endobronchial Watanabe spigot (EWS) (Novatech, Cedex, France), developed by Watanabe, is an effective method for endobronchial occlusion of lobar and segmental airways. It is relatively cheap and does not require specialized equipment such as guide wires or fluoroscopy as the spigot is placed under direct vision. This makes it an attractive option for resource-limited settings where treatment costs can be prohibitive. A recently published review cited a success rate of 85% with the use of spigots in BPF closure.[8]
In this case study, we describe a successful closure of a BPF resulting from TB in a newly diagnosed HIV-positive patient with concomitant gastric malignancy and severe wasting who had suffered a recent cardiac arrest. As of this writing, this is the first recorded case performed in Kenya and highlights a landmark procedure in the field of pulmonology in Kenya.
CASE REPORT
A newly diagnosed HIV-positive 57-year-old male presented to us in May 2024 with a 4-month history of progressively worsening pleuritic left-sided chest pain. He had no complaints of cough, chills, or night sweats. He reported poor appetite, early satiety, and unintentional weight loss. On examination, he was severely wasted and hypoxemic, requiring oxygen supplementation of 3L/min through nasal prongs.
Initial imaging included a high-resolution computed tomography (CT) scan of the chest, which demonstrated a complicated empyema with a partly trapped lung and patchy consolidation in all lung zones. A CT scan of the abdomen demonstrated an intraluminal mass arising from the gastric body, which was later biopsied endoscopically and confirmed to be a gastrointestinal stromal tumor.
A bronchoscopy and lavage were performed, which were positive for TB (Xpert-Rif assay). The patient was initiated on anti-TB treatment and broad-spectrum antibiotics. However, after consultation with the cardiothoracic surgeon, the patient underwent a thoracotomy and washout for clearance of the empyema. Intraoperatively, multiple locules of pus and necrotic debris were removed; however, the patient sustained a ventricular arrhythmia intraoperatively and a subsequent brief cardiac arrest with a successful resuscitation. Post-operatively, he required admission to the critical care unit with inotropic and ventilator support. The patient was extubated successfully with an actively bubbling chest tube in situ [Figure 1].

- Post-thoracotomy X-ray showing a residual left pneumothorax with a chest tube in place and bilateral patchy infiltrates.
A post-operative CT scan confirmed the presence of a persistent pneumothorax with a left lower lobe BPF and resolving consolidations [Figure 2]. The chest drain was left in situ for 2 weeks; however, the fistula did not spontaneously close, necessitating intervention. However, bronchoscopic intervention was deemed to be the safest option here due to the multiple comorbidities and high intraoperative risk with surgical repair.
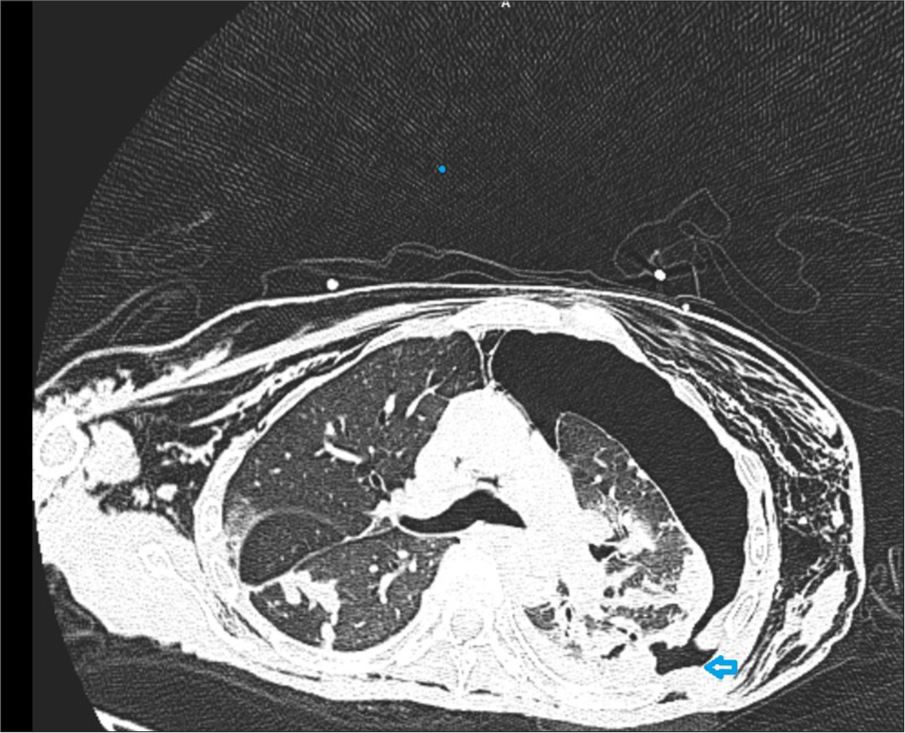
- Axial cut of a computed tomography chest showing a large left pneumothorax with an arrowhead pointing to the fistula location.
Closure of the BPF was done under total intravenous anesthesia, with a laryngeal mask airway used to maintain ventilation through the procedure. Localization of the BPF to the left superior segmental (LB6) bronchus was done using sequential balloon occlusion using a 6Fr endobronchial balloon. Occlusion of the LB6 bronchus led to a marked reduction in the chest drain bubbling. Retrograde instillation of methylene blue confirmed the location of the fistula as well [Figure 3]. A 7 mm spigot was then inserted and wedged in the LB6 bronchus using grasping forceps passed through the working channel of the bronchoscope (Fujifilm 2.88 mm working channel therapeutic scope). The size of the spigot was decided after visual assessment of the target bronchus, as well as an approximation using the inflated 6 Fr balloon. Cyanoacrylate glue was instilled through a catheter passed through the working channel of the bronchoscope to seal the spigot in place. Cyanoacrylate glue was used to prevent the dislodgment of the spigot. This was a concern due to the acute branching angle of the LB6 bronchus. The total procedure time was 60 min. Over the next 48 hours, the pneumothorax had reduced significantly, and bubbling had stopped.
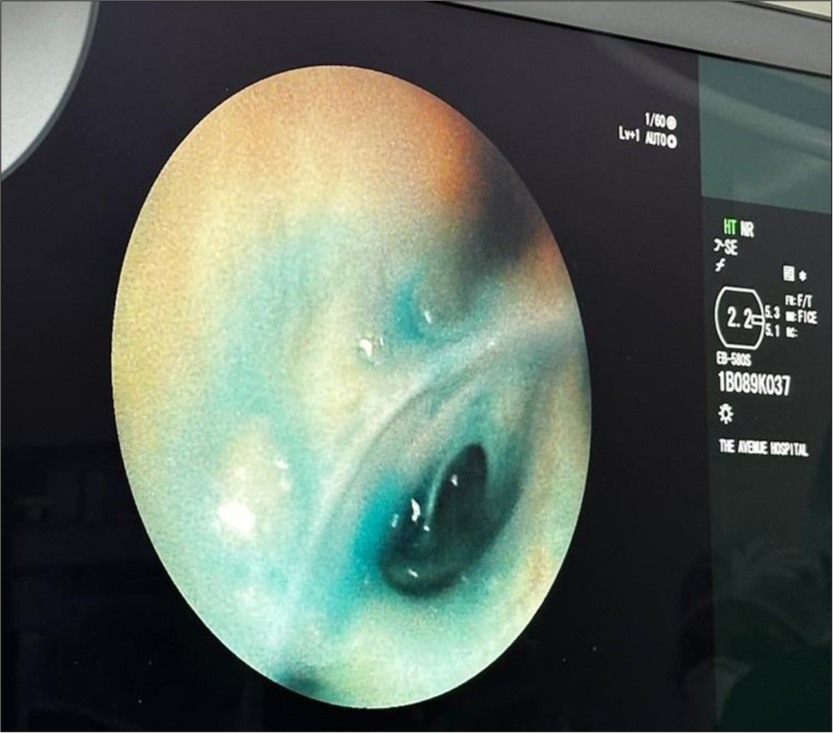
- Methylene blue dye leaking through the LB6 bronchus.
Unfortunately, 3 days after the procedure, the drain was observed to have increased in bubbling, and a chest X-ray showed the absence of the spigot that we believe the patient coughed out. A repeat bronchoscopy was performed with the placement of a smaller 6 mm spigot in LB6 with successful BPF closure [Figures 4 and 5]. A Heimlich valve was placed at the end of the chest drain tube to facilitate early ambulation, and the chest tube was removed 1-week post-spigot closure. The patient was subsequently discharged from the hospital and reviewed in the clinic. A follow-up X-ray showed a stable residual ex-vacuo pneumothorax with no interval change and spigot in place [Figure 6].
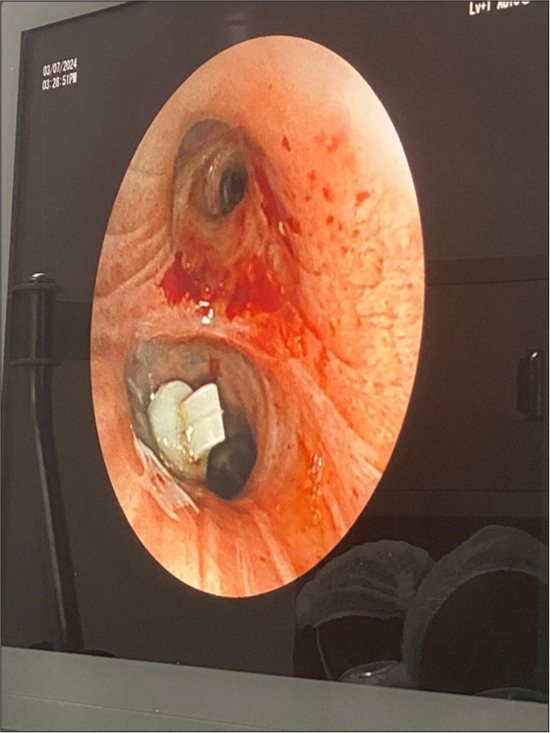
- Bronchoscopic view of spigot placement in LB6.
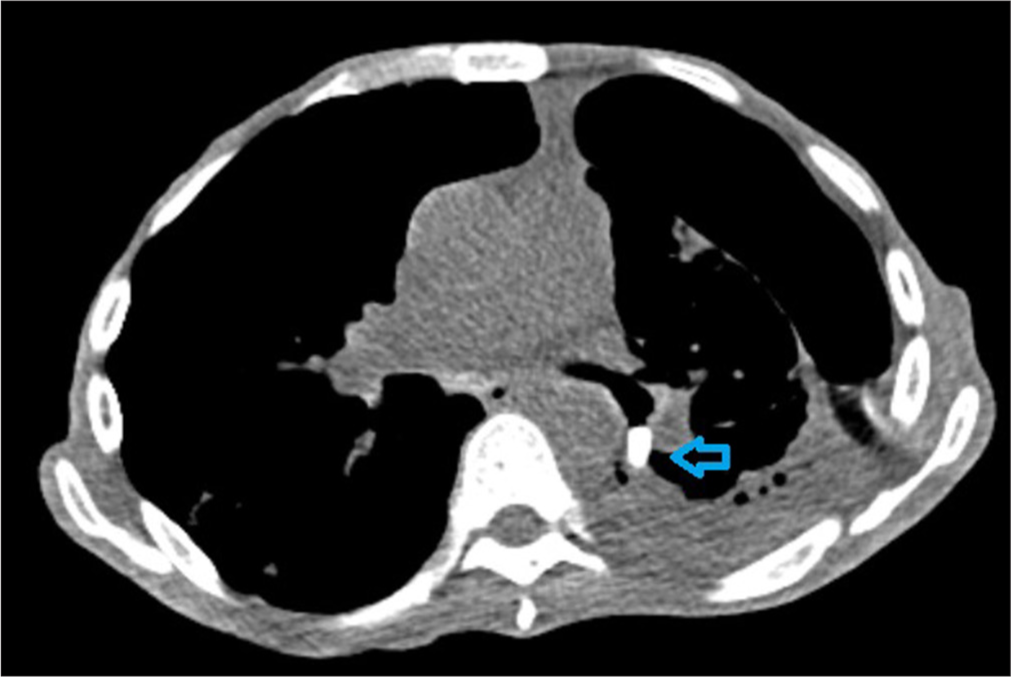
- Axial cut of a computed tomography chest showing the spigot in place in the LB6 bronchus (arrowhead).
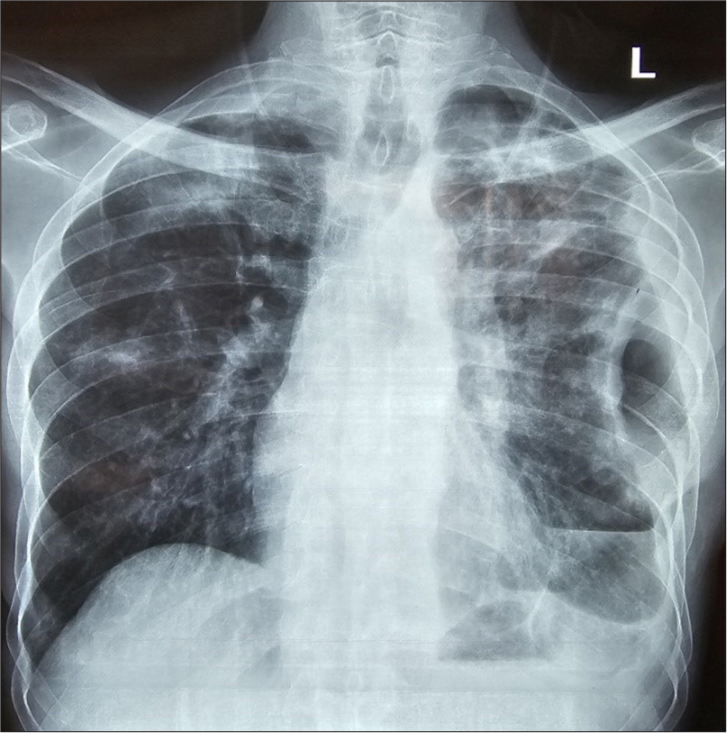
- Chest radiograph taken 3-week post-spigot placement. The chest drain has been removed and there is a significant reduction in the pneumothorax.
The second EWS was not removed as the patient declined any further interventions.
DISCUSSION
This case report highlights the efficacy and safety of EWS placement for BPF in a high-risk patient with multiple comorbidities and a recent cardiac arrest for whom surgical treatment was deemed to be too risky. The successful closure of the fistula allowed for the removal of the chest drain with improved patient morbidity and an earlier discharge than anticipated.
Despite being a highly useful procedure, EWS placement is not without its risks. Complications of spigot placement include dislodgment, migration, and bleeding. In our case, the 7 mm spigot got dislodged and needed a repeat procedure with a smaller spigot which could embed more snugly in the bronchus. Spigot placement is also a technically difficult procedure to perform, especially when navigating the spigot into a branching segmental bronchus.
There is also a risk of bronchoscope damage, especially when applying the glue, as it can cause permanent damage to the working channel if improperly done.
In Kenya, this was the first ever case of its kind, and some of the difficulties encountered included a lack of local availability of spigots and glue, which had to be imported in advance. This is an affordable procedure compared to valves, as the approximate cost of a spigot is US $70 and the cost of the valve is over US $1000. Interventional bronchoscopy is still a relatively new field with few hospitals able to offer advanced services. Performing this represents a huge stride in the budding field of interventional pulmonology, which will benefit many future patients.
CONCLUSION
Bronchoscopic spigot occlusion is a safe and effective procedure, especially in high-risk, non-surgical patients.
Acknowledgments:
The authors would like to thank Avenue Hospital, Nairobi, M.P. Shah Hospital, Nairobi, and Yashoda Hospital, Hyderabad, for their cooperation and dedication to patient care by supporting this project. We would also like to thank the patient for his cooperation and consent.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Management of complications after lung resection: Prolonged air leak and bronchopleural fistula. Thorac Surg Clin. 2020;30:347-58.
- [CrossRef] [PubMed] [Google Scholar]
- Kenya Tuberculosis Prevelence Survey 2016: Final Report. Ministry of Health 2018:50.
- [Google Scholar]
- Bronchopleural fistula after lobectomy for lung cancer: How to manage this life-threatening complication using both old and innovative solutions. Cancers (Basel). 2024;16:1146.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg. 1998;65:807-9.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic treatment of postoperative bronchopleural fistula: Experience with 45 cases. Ann Thorac Surg. 1998;66:923-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of endoscopic bronchial occlusion with an endobronchial Watanabe spigot for the management of intractable pneumothorax, pyothorax with bronchial fistula, and postoperative air leakage. Intern Med. 2020;59:1835-9.
- [CrossRef] [PubMed] [Google Scholar]
- Silver nitrate through flexible bronchoscope in the treatment of bronchopleural fistulae. J Thorac Cardiovasc Surg. 2009;138:603-7.
- [CrossRef] [PubMed] [Google Scholar]
- A simple method of bronchial occlusion with silicone spigots (endobronchial watanabe spigot; EWS®) using a curette. Ther Adv Respir Dis. 2016;10:518-24.
- [CrossRef] [PubMed] [Google Scholar]






