Translate this page into:
Bronchiectasis in African children: Prevalence, etiology, and clinical spectrum at a pediatric tertiary hospital in Cape Town, South Africa
*Corresponding author: Leah Githinji, Department of Pediatrics, Nelson Mandela University Gqeberha, South Africa.leahf@mandela.ac.za
-
Received: ,
Accepted: ,
How to cite this article: Mapani MK, Githinji L, Vanker A, Verwey C, Masekela R, Goga A, et al. Bronchiectasis in African children: Prevalence, etiology, and clinical spectrum at a pediatric tertiary hospital in Cape Town, South Africa. J Pan Afr Thorac Soc. 2024;5:135-42. doi: 10.25259/JPATS_17_2024
Abstract
Objectives:
The objective of the study was to describe the disease burden, etiology, and clinical spectrum of bronchiectasis in children attending a tertiary hospital in Cape Town, South Africa.
Materials and Methods:
Data were collected by chart review of all patients aged 3 months to 15 years attending the respiratory clinic at Red Cross War Memorial Children’s Hospital between January 2019 and December 2019. We included children who had a diagnosis of bronchiectasis based on a history of a recurrent (>3 episodes/year) or persistent (>4 weeks) wet or productive cough and a clinical phenotype characterized by any of the following: Exertion dyspnea, recurrent chest infections, growth failure, finger clubbing, and chest deformity associated with radiographic features of bronchiectasis on chest radiograph or chest tomography reported by a pediatric radiologist. Patients with cystic fibrosis were excluded from the study.
Results:
Of 337 children seen at the respiratory clinic during the study period, 58 (17.2%) had bronchiectasis that was diagnosed at a mean age of 34 months (standard deviation 26), and 32 (55.0%) were female. The most common causes of bronchiectasis were post-infectious 25 (43.1%), and underlying immunodeficiencies 19 (32.8%), including 16/58 (27.6%) who were living with human immunodeficiency virus (HIV) and 3 (5.1%) with primary immunodeficiency. Other causes included aspiration syndrome 8 (13.8%) and anatomical abnormalities 4 (6.9%). Of the participants with post-infectious bronchiectasis, tuberculosis (TB) was the most common organism isolated 16 (64.0%), and most common in children living with HIV (11/16, 68.8%). Cough was common in 48 (82.8%), with wet cough being predominant in 41 (85.4%), course crepitations were found in 37 (63.8%), hyperinflation in 24 (41.4%), finger clubbing in 21 (36.2%), wheeze in 16 (29.3%), and exertional dyspnea in 7 (12.0%).
Conclusion:
Bronchiectasis is common in South African children, usually resulting from previous pneumonia episodes, with TB being the most common infective cause. The importance of early diagnosis and treatment of underlying causes, especially infectious diseases in low-middle-income settings, to prevent bronchiectasis is highlighted.
Keywords
Bronchiectasis
Children
Etiology
Burden
“Clinical spectrum”
INTRODUCTION
Bronchiectasis is a respiratory disease characterized by irreversible airway damage.[1] The cardinal features of bronchiectasis include stasis of infected airway secretions, regional or diffuse airway wall dilation, thickening, and destruction with loss of structural integrity. The associated prolonged neutrophilic inflammatory and secretory response within the airways cause a chronic or recurrent “wet” cough in young children and mucopurulent sputum expectoration in older children. This clinical phenotype, coupled with radiological findings of airway wall thickening and dilatation on chest computerized tomography (CT), is the criteria for making a diagnosis of bronchiectasis.[2-4]
Bronchiectasis lies at the end of a continuum of suppurative airway inflammation that ranges from an inconsequential protracted bacterial bronchitis to chronic suppuration that leads to irreversible airway damage and results from different disease processes or insults.[5,6] These include diseases that impair mucociliary clearance (e.g., primary ciliary dyskinesia [PCD] and cystic fibrosis [CF]), altered immune responses (e.g., primary or acquired immunodeficiency, chronic infections), structural abnormalities of the airways (e.g., bronchomalacia, trachea-esophageal fistula, and bronchomegaly), or disease associated with systemic inflammation. Similarly, events that result in chronic inflammation and airway damage, such as recurrent lower respiratory tract illness, foreign body aspiration, or recurrent aspiration, may cause bronchiectasis.[7]
The global burden of bronchiectasis has increased over the past decades despite improved living conditions and access to antibiotics and medical facilities.[8] Further, it has now been recognized that there is a global increase in the prevalence of bronchiectasis with associated morbidity and mortality, especially in children living with social deprivation.[9-11] The global prevalence of bronchiectasis is reported to range from 0.7/1000 to 14.7/1000 in children <15 years of age.[10] The true global pediatric prevalence is difficult to determine due to diagnostic criteria needing chest CT, which is not easily accessible in low- to middle-income settings. Hence, there remains a paucity of data from these settings and minimal data from Africa despite the high burden of pediatric chronic lung disease.[9]
Management of bronchiectasis in our setting mirrors global guidelines[12] which include airway clearance, sputum surveillance, treatment of acute exacerbations with antibiotics, and regular follow-up at our pediatric respiratory outpatient clinic.
Current evidence suggests that early diagnosis and treatment improves outcomes. However, delayed diagnosis remains common and mainly arises from low awareness of bronchiectasis among clinicians and limited access to diagnostic tools.[13,14] Further, there is evidence that the majority of adult lung disease has its origins in childhood.[15,16] It is hence important that we improve our knowledge of bronchiectasis in African children, where risk factors for disease are common and may differ from other settings. This would facilitate the development of effective prevention, diagnostic, and management strategies with the potential to improve the trajectory of the disease.
This study aimed to describe the burden of disease, etiology, and clinical spectrum of bronchiectasis, not including CF, in children attending a tertiary pediatric respiratory service at Red Cross War Memorial Children’s Hospital (RCWMCH) in Cape Town, South Africa.
MATERIALS AND METHODS
Study design and inclusion criteria
This was a retrospective cross-sectional study undertaken at RCWMCH, Cape Town, South Africa. A chart review of children diagnosed with bronchiectasis attending the respiratory clinic at RCWMCH between January and December 2019 was conducted. Children between 3 months and 15 years of age who attended the clinic were included if the clinical records indicated that they had a diagnosis of bronchiectasis based on:
History of a recurrent (>3 episodes/year) or persistent (>4 weeks) productive or wet cough
A clinical phenotype characterized by any of the following: exertion dyspnea, recurrent chest infections, growth failure, finger clubbing, and chest deformity) and
Characteristic radiographic features of bronchiectasis on plain chest radiograph or chest CT reported by a pediatric radiologist. The radiographic features included tram-tracking or ring shadows on either CT or chest radiographs.
Patients with bronchiectasis related to CF were excluded from this study.
Ethics approval
Ethical approval and authority to waiver informed consent for the study was granted by the Human Research Ethics Committee, University of Cape Town (HREC 179-2020).
Data collection
Data were collected retrospectively from the patient clinic files using the pre-designed study case report form (CRF). Patient demographic and nutritional status details using WHO reference values,[17] medical and family history, clinical details at diagnosis, etiology of bronchiectasis, and most recent clinical details at the time of study enrolment were collected as documented in the clinical notes during admission to hospital and during follow-up in clinic. As part of our clinic protocol for these patients, lung function testing is usually done 1–2 times per year. Sputum surveillance is done during an exacerbation or during a routine clinic visit, which is 6 monthly if there are no acute exacerbations in between.
Participant’s most recent clinical symptoms and signs were collected from the last visit in 2019, and a review of all the clinic visits during 2019, including past lung function measures. Radiology and laboratory (including histology) results were recorded from the radiology online portal and the National Health Laboratory Service system, respectively. Plain chest radiographs and CT scans were reported by a pediatric radiologist and categorized as a normal, diffuse, or focal disease: dilated and thick airway walls, non-tapering airways, presence of tram tracks, and “signet ring” sign. Lung function was measured with spirometry and bronchodilator response (BDR) testing. Testing was done using a CareFusion spirometer (MS Pneumoscope, Jaeger-CareFusion, Germany) by a trained respiratory technologist and following international guidelines.[18] The first ever spirometry result obtained since admission to the respiratory clinic and the last spirometry results obtained in 2019 were collected. Measures included the forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio. BDR was measured after repeating the test 15 min after 400 mcg of salbutamol was administered through the spacer. The Quanjer GLI 2012 reference equation was used to interpret tests.[19] In patients who had a bronchoscopy done, findings were collected.
Data analysis
Data were entered into a Redcap database from the CRF. Descriptive statistics were used to describe the characteristics of the study population, clinical signs, symptoms, and disease severity. For normally distributed data, mean and standard deviation (SD) were used. The burden of bronchiectasis among the study population was expressed as count (percent), the proportion of patients with bronchiectasis against total patients in 2019. Summary proportions were used to express the etiology of bronchiectasis.
RESULTS
Disease burden
There were a total of 627 clinic attendances (including repeat visits) for the period January to December 2019. A total of 337 patients attended the clinic. A total of 58 patients met the inclusion criteria for the study, giving a clinic prevalence for bronchiectasis of 17.2%, as shown in Figure 1. The diagnosis of bronchiectasis was based on chest radiographs for 30 (53.4%) and on CT in 28 (46.6%) participants, as shown in as shown in Table 1.
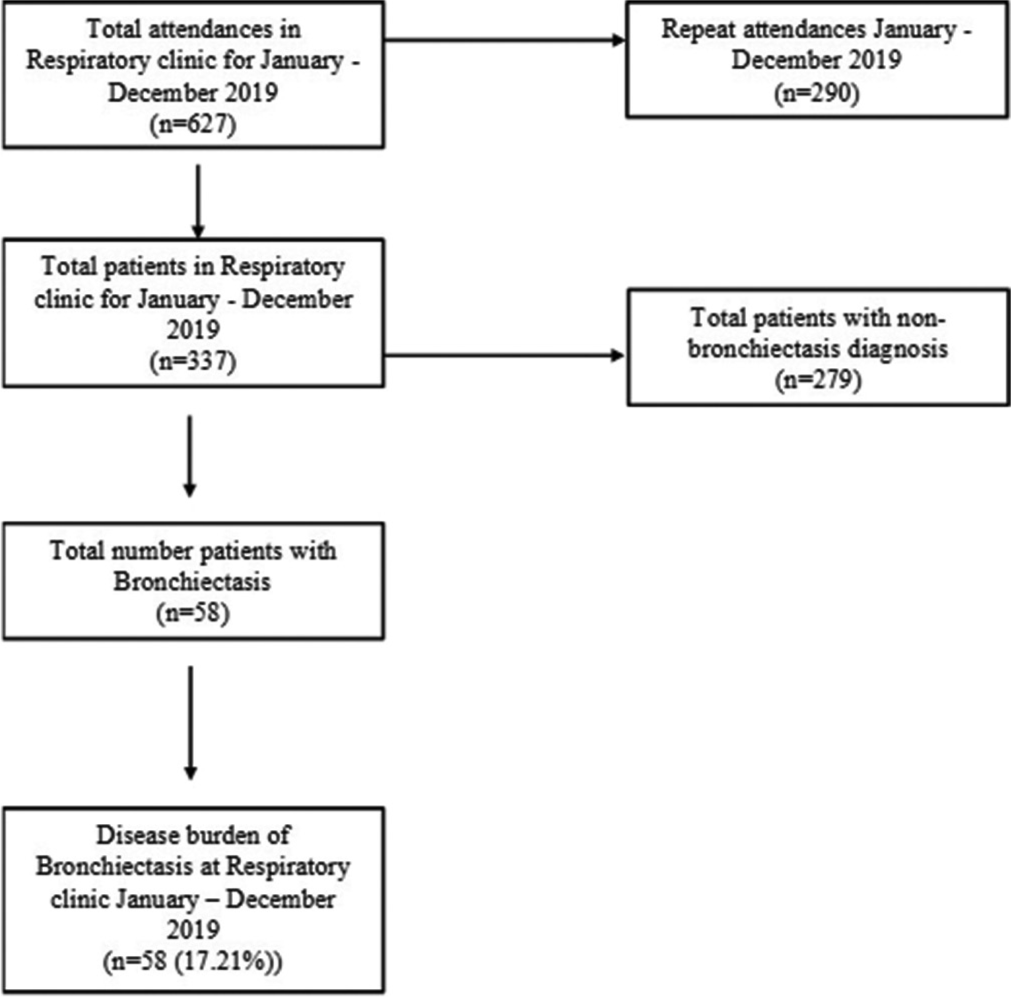
- The protocol that was used to determine the disease burden in the respiratory clinic. A total of 337 patients attended the clinic between January and December 2019 and 58 had bronchiectasis, amounting to 17.2% disease burden.
| Patient characteristic | n (%) |
|---|---|
| Sex (number, %) | |
| Female | 32 (55.2) |
| Male | 26 (44.8) |
| Age (months) at enrolment (Mean, SD) | 92.2 (41.0) |
| Males | 95.5 (47.9) |
| Females | 89.3 (34.7) |
| Age (months) at diagnosis (Mean, SD) | 34.2 (26.3) |
| Males | 34.7 (31.6) |
| Females | 33.9 (22.8) |
| HIV status (number, %) | |
| HIV unexposed and uninfected | 36 (62.0) |
| Positive | 16 (28.0) |
| HEU | 6 (10.0) |
| Gestation age at birth in weeks (mean SD) | 37.5 (1.3) |
| Term (≥37 weeks) | 53 (91.4) |
| Preterm (Below 37 weeks) | 8 (13.8) |
| Received oxygen therapy at birth (number, %) | 5 (9) |
| WHZ*, Mean (SD) | 0.0 (1.6) |
| HAZ*, Mean (SD) | 1.2 (1.6) |
| Number of chest infections ever, mean (SD) | 2 (1.2) |
| Number of admissions ever, mean (SD) | 2 (0.9) |
| Radiology basis for bronchiectasis diagnosis (number, %) | |
| Chest X-ray | 30 (51.7) |
| HRCT | 28 (48.3) |
Demographic and clinical characteristics
Cohort demographics are presented in Table 1. The mean (SD) age at enrolment was 92.2 (41.0) months. Most patients, 50 (86.0%), were older than 5 years and 32 (55%) were female. The mean (SD) age at diagnosis was 34.2 (26.3) months, with over a third, 21 (36%), diagnosed before 24 months. Further, the mean age at diagnosis for males was 34.7 (31.6) and 33.9 (22.8) for females. Nutritional status was normal for most, with mean (SD) weight for height z-score of 0.0 (1.6). The mean (SD) height for age z-score was 1.2 (1.6). None were stunted, underweight, or obese. The gestational age at birth ranged from 32 to 40 weeks, mean (SD) 37.5 (1.3); only 8 (13.8%) were pre-term, and none were early preterm (<32 weeks). Sixteen (27.6%) were living with human immunodeficiency virus (HIV), and 6 (10.3%) were HIV-exposed but uninfected. All children had a history of at least one previous lower respiratory tract infection (LRTI), with a mean of 2 (SD 1.2) events, including an average of 2 (SD 0.9) hospital admissions ever for LRTI per child. Diagnostic workup included bronchoscopies in 34 (58.6%) of children, 3 (5.2%) lung biopsies, and 23 (39.7%) upper gastrointestinal tract scintigraphy (milk scan).
Bronchiectasis etiology
The most common cause of bronchiectasis in this study was post-infectious bronchiectasis in 25 (43.1%) children, as shown in Table 2. Nineteen (32.8%) children had underlying immunodeficiencies; 16/58 (27.6%) had HIV disease; and 3/58 (5.2%) had primary immunodeficiency (PID). Other causes included gastroesophageal reflux disease (GORD) and aspiration syndrome in 8 (13.8%), airway anatomical abnormalities in 4 (6.9%), and 2 (3.4%) participants had bronchiectasis of unknown cause, as shown in Table 2.
| Cause of bronchiectasis | n(%) |
|---|---|
| 1. Post-infectious | 25 (43.1) |
| 2. Immunodeficiency i) HIV ii) Primary immune deficiency a. Agammaglobulinemia b. Immunoglobulin A deficiency |
19 (32.8) 16 (84.2) 3 (15.8) 1 (33.3) 2 (66.7) |
| 3. GORD and aspiration | 8 (13.8) |
| 4. Airway anatomy abnormalities: a. Right-sided distal tracheobronchomalacia b. Right upper lobe branching abnormality c. CPAM Type 1 d. Tracheoesophageal fistula repair |
4 (6.9) 1 (1.7) 1 (1.7) 1 (1.7) 1 (1.7) |
| 5. Bronchiectasis of unknown cause | 2 (3.4) |
GORD: Gastroesophageal reflux disease, CPAM: Congenital pulmonary adenomatoid malformation, HIV: Human immunodeficiency virus
Among the children with post-infectious bronchiectasis, the most common responsible organism was Mycobacterium tuberculosis (TB), 16/25 (64.0%), followed by adenovirus, 8/24 (37.5%). Eleven of 16 (68.8%) HIV-infected children had post-TB bronchiectasis and two had post-adenovirus bronchiectasis. Microbiological coinfection was common among all participants, as shown in Table 3.
| HIV-infected n=16 |
HIV-exposed uninfected n=6 |
No HIV exposure/infection n=36 |
|
|---|---|---|---|
| Mycobacterium tuberculosis | 11 | 0 | 5 |
| Adenovirus | 1 | 1 | 7 |
| Coinfection | 4 | 5 | 24 |
Coinfection organisms: Adenovirus, Human metapneumovirus, Human papillomavirus, Respiratory syncytial virus, Enterovirus, Moraxella catarrhalis, Haemophilus Influenza, Corona Virus, Human rhinovirus, HIV: Human immunodeficiency virus
Clinical spectrum, imaging, and lung function
Children had a mean (SD) of 2 (1.1) clinic visits during the year, with nearly a quarter (22%) having more than two visits. The most common reported symptom was cough in 48/58 (82.8%), of which 42 (87.5%) described as wet cough, 4 (8.3%) both wet and dry cough, and 2 (4.1%) as dry. Nineteen (39.6%) reported coughing multiple times daily, while 16 (33.3%) reported having a cough only when sick, as shown in Table 4. Continuous sputum production was reported by 20/48 (41.7%), 13 (27.1%) reported sputum production only when ill, and 12 (25.0%) reported seldom coughing, as shown in Table 4. Ten (17.2%) participants did not have a cough during 2019. Other reported symptoms were exertional dyspnea in 7/58 (12.1%) and wheezing in 17/58 (29.3%), of which over half of the participants, 9/17 (52.9%), wheezed multiple times daily. Twenty-three (39.7%) participants had one exacerbation in 2019, while 12 (20.6%) had 2 or more exacerbations. Twenty-three (39.7%) participants had no exacerbations in 2019. Females were more likely to have had more than one exacerbation compared to male children (P = 0.002). Only 2 (3.4%) participants were on home oxygen during the review period; both were on oxygen in the initial 6 months after diagnosis and were weaned off by the end of 2019. Thirty-six (62.1%) children had finger clubbing; 37 (64.0%) had coarse crepitations on auscultation, as shown in Table 4.
| Clinical feature | Number (%) |
|---|---|
| Cough | 48 (82.8) |
| Cough character (n=48) | |
| Wet | 42 (72.4) |
| Dry | 2 (3.4)) |
| Both wet and dry | 4 (6.9) |
| Cough frequency (n=48) | |
| Multiple times/day Only when sick SeldomOnce/day | 19 (39.6) |
| Only when sick | 16 (33.3) |
| Seldom | 12 (25.0) |
| Once/day | 1 (2.1) |
| Sputum production (n=48) | |
| Continuously | 20 (41.7) |
| Only when sick | 13 (27.1) |
| Seldom | 12 (25.0) |
| None | 3 (6.2) |
| Wheeze ever | 16 (27.6) |
| Exertional dyspnea (self-reported) | 7 (12.1) |
| Crepitations | 37 (63.8) |
| Number of exacerbations in 2019 | |
| One | 23 (39.7) |
| Two | 10 (17.2) |
| Three and more | 2 (3.4) |
| No exacerbations in 2019 | 23 (39.7) |
| Hyperinflation of the lungs | 24 (41.4) |
| Home oxygen use | 2 (3.4) |
| Finger clubbing | 21 (36.2) |
| Baseline chest X-ray (n=58) | |
| Focal disease | 11 (19.0) |
| Diffuse disease | 47 (81.0) |
| Sputum Culture Results (last sputum surveillance 2019, n=54, 93%) | |
| Normal respiratory flora | 38 (70.4) |
| No growth | 7 (13.0) |
| Mycobacterium tuberculosis | 2 (3.7) |
| Bordetella pertussis | 1 (1.9) |
| Candida species | 1 (1.9) |
| Hemophilus influenzae | 1 (1.9) |
| Moraxella catarrhalis | 1 (1.9) |
| Staphylococcus aureus | 1 (1.9) |
| Pseudomonas aeruginosa | 1 (1.9) |
| Pseudomonas aeruginosa mixed with M. catarrhalis | 1 (1.9) |
| No sputum submitted in 2019 | 4 (6.9) |
| Viral panel (n=32) | |
| Adenovirus | 3 (9.4) |
| Bocavirus | 1 (3.1) |
| Enterovirus | 1 (3.1) |
| Human metapneumovirus | 1 (3.1) |
| Rhinovirus | 1 (3.1) |
| Respiratory Syncytial Virus | 2 (6.2) |
| Influenza B | 1 (3.1) |
| Parainfluenza | 1 (3.1) |
| Negative | 21 (65.6) |
| No test done | 26 (44.8) |
| Bronchoscopy n=34 | |
| Normal anatomy | 30 (88) |
| Abnormal branching of the RUL bronchus | 1 (3) |
| Extensive papillomatous disease of trachea, | 1 (3) |
| bronchi | 1 (3) |
| Granulation tissue in the left main bronchus | 1 (3) |
| Tracheobronchomalacia right side | |
| Lung Biopsy n=3 | |
| Chronic lung inflammation | 1 (1.7) |
| CPAM type 1 | 1 (1.7) |
| Lymphocytic interstitial pneumonia | 1 (1.7) |
CPAM: Congenital pulmonary adenomatoid malformation, RUL: Right upper lobe
Baseline chest X-ray at admission to respiratory clinic showed diffuse lung disease in 48 (82.8%) and focal lung disease in 10 (17.2%), as shown in Table 4. Most children, 54 (93.1%), had sputum surveillance culture results in 2019. Of these, 38 (70.4%) grew normal respiratory flora, 2 (3.7%) cultured MTB, and 7 (13.0 %) had no growth. Other organisms isolated, representing 1 (1.9%) participant each, included Bordetella pertussis, Parainfluenza, Moraxella catarrhalis, Staphylococcus aureus, Pseudomonas aeruginosa, and Candida species, and a mixed growth of P. aeruginosa and M. catarrhalis. The predominant virus isolated was adenovirus in 3 (9.4%) and RSV in 2 (6.2%) of participants. Other viruses isolated occurred as mixed growth, as shown in Table 4.
Thirty-three (56.9%) children had spirometry results of baseline tests after enrolment, and the last test was done in 2019, as shown in Table 5. Mean (SD) time interval between tests was 60 (3.2) months. This included the FEV1, FVC, and the ratio of FEV1/FVC. The mean (SD) z-score for FEV1 and FVC improved over the 12 months, with FEV1 z score −4.3 (1.1) at enrolment and −3.9 (1.0) at the last test in 2019 (P < 0.001), and FVC-z score was −3.8 (1.4) and −3.1 (1.0) at baseline and the last test, as shown in Table 5.
| Mean (SD) Z score Baseline | Mean (SD) z-score Latest | P-value | |
|---|---|---|---|
| FEV1 | −4.3 (1.0) | −3.9 (1.0) | 0.0003 |
| FVC | −3.8 (1.4) | −3.1 (1.0) | 0.0002 |
| FEV1/FVC | −1.83 (1.9) | −2.0 (1.8) | 0.19 |
The table shows comparison of first-ever and last spirometry of 2019 results. FEV1: Forced expiratory volume in the 1st s. FVC: Forced vital capacity of first-ever spirometry test done for patient, SD: Standard deviation
DISCUSSION
Bronchiectasis is an important cause of chronic lung disease in children. We found 17.2% of all patients attending our tertiary respiratory clinic with bronchiectasis. The most common cause of bronchiectasis in our study population was post-infectious; all children had a history of recurrent LRTIs. The most common cause of LRTI was TB and adenovirus, which differed by HIV status. This highlights the importance of strengthening diagnostic pathways for children at risk of bronchiectasis and pneumonia preventive strategies for all children. Chronic respiratory symptoms were common, with mean lung function of the children severely impaired over the study period. This study adds to the knowledge of bronchiectasis in African children.
Bronchiectasis prevalence was 17% in our tertiary respiratory clinic in the setting of a high HIV endemicity, with nearly 30% of all children in the clinic with HIV infection. A study in Zimbabwe of 84 adolescents living with HIV with abnormal HRCT, with a median duration of ART of 4.7 years, had a higher bronchiectasis prevalence of 33%.[20] Studies in high-income countries, in contrast, found a much lower prevalence, ranging from 0.2–2.3/100,000 in European countries and 1.5/100,000 in New Zealand people of European descent. In disadvantaged and indigenous communities in New Zealand, however, the prevalence was higher: 15/100,000 and 17.8–18.3/100,000 population in Pacific islanders.[9] There are no comparable population-level statistics in low-middle income settings with high HIV prevalence; however, we estimate that the high burden in our clinic population (172/1000 clinic attendees) reflects both the selected population of children with CLD and the setting of high-risk factors for bronchiectasis, notably TB, HIV, and high infective exposure. Community prevalence data from South Africa are needed.
Although the most common cause of bronchiectasis in our participants was attributed to post-infectious causes, nearly a quarter of our children had immune deficiencies, mainly arising from perinatally acquired HIV. These children all had a history of recurrent or severe LRTI. TB is endemic in South Africa, with 322 cases (95% CI 230–428) per 100,000 population[21] and not surprising that it was the most common cause identified in children living with HIV (68.8%) and 19.3% in HIV uninfected children, highlighting the need for TB prevention. Adenovirus has been found to cause long-term sequelae, including bronchiectasis, following respiratory infections as a single infection or coinfection with other organisms.[22,23] This is similar to our findings, where adenovirus was isolated as a single organism as well as a coinfection. The most common cause of bronchiectasis among indigenous poor communities in Australia was reported to be post-infectious/idiopathic (94.5%),[24] similar to our results although our cohort was hospital-based. Other common causes, such as GORD, PID, and congenital or acquired airway anatomical abnormalities, are antecedents to recurrent respiratory infections, which may further result in bronchiectasis. PCD and PID have been recognized to be common among societies with a high frequency of consanguinity, as described in Turkey,[25] while the incidence of CF, and PCD are unknown in many low- or middle-income countries (LMIC) due to limited diagnostic capacity.[1,24] Determination of the etiology of bronchiectasis is important and key to the successful management of the disease.
Our children were younger at diagnosis (mean age 34 months) compared to children in New Zealand (median 5.4 years).[10] Unlike in our cohort, where the cause was mainly infections, diagnosis of bronchiectasis in infancy has been associated with congenital lung malformation and other genetic diseases like PCD.[6,26] The diagnosis of bronchiectasis in our cohort was made early, and this demonstrates that children who are at risk of bronchiectasis may present early and should be screened for bronchiectasis.
Similar to previous studies, chronic wet cough was the most common symptom.[1] Other researchers have reported 28– 100% chronic wet cough in children from LMICs.[1] Cough is the earliest and most important symptom to recognize and investigate in children with suspected bronchiectasis.[9] Not recognizing this causes delays in diagnosis and treatment and has a negative impact on prognosis, increasing spending on national health budgets and family expenditures. Increasing productive cough is a sign of exacerbation and requires escalation of management interventions. Therefore, strengthening prevention, early diagnosis, and care has the potential to mitigate these untoward effects.
Wheeze was present in a quarter (27.6%) of our cohort, which was similar to findings in Tunisia (20%); the United Kingdom reported a lower prevalence of wheezing (10%), and Alaskan children had a high prevalence of wheezing (41%) which was attributed to RSV hospitalization.[10] In our cohort, RSV was isolated as a coinfection in two HIV-negative children. The finding of wheezing is variable among different studies and needs further evaluation to determine the true prevalence and the underlying risks.
Features of severe bronchiectasis in children may include clinical features such as exercise intolerance, frequent exacerbations, hemoptysis, frequent attendances at clinics, failure to thrive, reduced lung function, use of home oxygen, and presence of finger clubbing.[1,24,27] These features were not predominant in our cohort. Early diagnosis in our cohort and access to appropriate care may be responsible for this.
Diffuse or multilobar disease on the baseline chest radiograph was predominant compared to focal disease, which is sometimes used as a marker of disease severity. Focal disease may be associated with foreign body aspiration, though our cohort did not have that history. Despite this preponderance of diffuse disease on the baseline chest radiograph, our cohort did not have predominant severe disease. Chest radiograph changes in our patients were persistent, and thus, chest CT was not offered. In addition, it is not always feasible to recommend chest CT for all patients to diagnose bronchiectasis in LMICs as is recommended in high-income countries. This may have limited our ability to diagnose all patients with bronchiectasis. We, however, highlight the need for earlier diagnosis to prevent this extent of disease, and chest CT plays an important role in the detection of early disease and can facilitate targeted investigations and treatment.
Sputum surveillance results had few children with H. influenzae or H. parainfluenza, which were the predominant organisms in LMIC settings in children living with HIV.[28] H. influenzae accounted for 47% of children having exacerbations in Australia.[24] TB was isolated on surveillance sputum done in 2019 and can easily cause opportunistic infections in patients with bronchiectasis. Our cohort was on prophylactic azithromycin, which may be responsible for low bacterial load in sputum.[9,29] BrookeHollidge et al. reported that low pathogen load in sputum was associated with the mildest form of the disease.[30]
Although the mean z-FEV1 and z-FVC showed severe dysfunction of >−3SD, our cohort showed lung function improvement between baseline and last test, probably due to physiological growth and improved health care as all children were being regularly followed up in a tertiary hospital in Cape Town. This ensured the children could get regular airway clearance, sputum surveillance, and prompt treatment of exacerbations.
This is one of the first studies to describe the etiology and clinical spectrum of bronchiectasis in South African children attending a general respiratory clinic. The strength included access to comprehensive clinical information and investigations. Limitations of the study include being a retrospective study; we relied on data from clinical records. Although these were available for all children and had been collected by the same team of experienced pulmonologists, there may be reporting bias. Further, there could have been a selection bias of children with severe disease attending a specialist respiratory clinic in a tertiary facility. Further, the research was conducted in a tertiary center and, so unlikely to reflect the general population. Further data are needed to describe disease burden, etiology, and clinical spectrum at the community level.
Take home message
Data on bronchiectasis in African settings are lacking. Bronchiectasis was common in children attending a tertiary Hospital in Cape Town, South Africa, the majority of whom presented with chronic cough. Post-infectious causes were predominant, especially pulmonary tuberculosis in children living with HIV. Preventative strategies to curb pneumonia, tuberculosis, and HIV exposure are key to mitigating bronchiectasis in low-to-middle-income settings.
CONCLUSION
Bronchiectasis is common in our tertiary respiratory clinic setting and an important cause of lung morbidity in children. Post-infectious causes are the most predominant due to the high burden of early respiratory childhood infections in this setting. Post-TB bronchiectasis in children, including those living with HIV, is an important cause in TB-endemic settings and is the leading cause of bronchiectasis in children living with HIV. Prevention, early diagnosis, and management of TB in children can prevent the development of bronchiectasis. Clinical features of bronchiectasis vary depending on the extent of the disease. Chronic wet cough remains the hallmark clinical feature of bronchiectasis and any such cough in children should be evaluated further to facilitate early diagnosis and early intervention.
Acknowledgment
The authors are grateful to the patients whose records were used to extract data to make this study possible and to the pulmonology unit at Red Cross War Memorial Children’s Hospital (RCWMCH), including respiratory technicians, nurses, and registry clerks who tirelessly contributed to this study. We acknowledge the Bronchiectasis in African Children, Prevalence, Etiology, and Clinical Outcome collaboration that established the registry.
Ethical approval
The research/study was approved by the Institutional Review Board at the University of Cape Town Human Research Ethics Committee, number HREC179-2020, dated 2nd January 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Bronchiectasis in children: Diagnosis and treatment. Lancet. 2018;392:866-79.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchiectasis in children. Pediatr Clin North Am. 2009;56:157-71.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchiectasis in Germany: A population-based estimation of disease prevalence. Eur Respir J. 2015;46:1805-7.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchiectasis: How to be an orphan with many parents? Eur Respir J. 2016;47:10-3.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol. 2008;43:519-31.
- [CrossRef] [PubMed] [Google Scholar]
- Kendig and Chernick's disorders of the respiratory tract in children (8th ed). Philadelphia, PA: Elsevier/Saunders; 2012.
- [Google Scholar]
- British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:i1-58.
- [CrossRef] [PubMed] [Google Scholar]
- Quality standards for managing children and adolescents with bronchiectasis: An international consensus. Breathe (Sheff). 2022;18:220144.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front Pediatr. 2017;5:27.
- [CrossRef] [Google Scholar]
- Indigenous children from three countries with non-cystic fibrosis chronic suppurative lung disease/bronchiectasis. Pediatr Pulmonol. 2014;49:189-200.
- [CrossRef] [PubMed] [Google Scholar]
- Non-CF bronchiectasis: Clinical and HRCT evaluation. Pediatr Pulmonol. 2003;35:477-83.
- [CrossRef] [PubMed] [Google Scholar]
- European respiratory society guidelines for the management of children and adolescents with bronchiectasis. Eur Respir J. 2021;58:2002990.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal pulmonary function of childhood bronchiectasis and comparison with cystic fibrosis. Thorax. 2006;61:414-8.
- [CrossRef] [PubMed] [Google Scholar]
- Differences and similarities in noncystic fibrosis bronchiectasis between developing and affluent countries. Paediatr Respir Rev. 2010;12:91-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology, causes and genetics of paediatric and adult bronchiectasis. Respirology. 2019;24:1053-62.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypes of adult bronchiectasis: Onset of productive cough in childhood and adulthood. COPD. 2009;6:130-6.
- [CrossRef] [PubMed] [Google Scholar]
- WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-forage: Methods and development Switzerland: World Health Organization; 2006.
- [Google Scholar]
- Standardization of spirometry 2019 Update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200:e70-88.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur Respir Soc. 2012;40:1324-43.
- [CrossRef] [PubMed] [Google Scholar]
- Human immunodeficiency virus-associated chronic lung disease in children and adolescents in Zimbabwe: Chest radiographic and high-resolution computed tomographic findings. Clin Infect Dis. 2018;66:274-81.
- [CrossRef] [PubMed] [Google Scholar]
- Geneva: World Health Organization. 2020. Available from: https://www.who.int/publications/i/item/9789240013131 [Last accessed on 2023 Jun 23]
- [Google Scholar]
- Bronchiectasis and bronchiolitis obliterans post respiratory syncytial virus infection: Think again. J Paediatr Child Health. 1999;35:497-8.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic lung damage caused by adenovirus type 7: A ten-year follow-up study. Chest. 1981;80:127-31.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with “Frequent Exacerbator” phenotype in children with bronchiectasis: The first report on children from the Australian Bronchiectasis Registry. Respir Med. 2021;188:106627.
- [CrossRef] [PubMed] [Google Scholar]
- A review of the etiology and clinical presentation of non-cystic fibrosis bronchiectasis: A tertiary care experience. Respir Med. 2018;137:35-9.
- [CrossRef] [PubMed] [Google Scholar]
- The etiologies of non-CF bronchiectasis in childhood: A systematic review of 989 subjects. BMC Pediatr. 2014;14:4.
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge, attitudes and practices of general practitioners about asthma in the city of Ouagadougou. Le Mali Med. 2012;27:10-3.
- [Google Scholar]
- Lack of efficacy of erythromycin in children with human immunodeficiency virus-related bronchiectasis-A randomised controlled trial. Paediatr Respir Rev. 2013;14:S82.
- [CrossRef] [Google Scholar]
- Pediatric bronchiectasis: No longer an orphan disease. Pediatr Pulmonol. 2016;51:450-69.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in non-cystic fibrosis bronchiectasis severity and bacterial load: The potential role of hormones. Ther Adv Respir Dis. 2021;15:1-9.
- [CrossRef] [PubMed] [Google Scholar]







