Translate this page into:
Asthma control among teenagers attending the respiratory outpatient clinic of an academic hospital in Pretoria, South Africa
*Corresponding author: Matlawene John Mpe, Department of Medicine, Sefako Makgatho Health Sciences University, Pretoria, Gauteng, South Africa. oupampe@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garrach B, Kalidas K, Mpe MJ. Asthma control among teenagers attending the respiratory outpatient clinic of an academic hospital in Pretoria, South Africa. J Pan Afr Thorac Soc. 2024;5:62-8. doi: 10.25259/JPATS_4_2024
Abstract
Objectives:
Bronchial asthma is characteristically a diverse illness that results from chronically inflamed bronchi. Adolescents are a particularly vulnerable group to asthma, and both its prevalence and death rate have increased in this patient population. Asthma care creates a particular set of difficulties for adolescents who are at a stage where there is a search for greater autonomy and changing social and emotional dynamics. The aim of this study was to describe the clinical characteristics of teenage asthmatics in an academic hospital.
Materials and Methods:
This was a cross-sectional study of patients between the ages of 13 and 18 who had consented to participate. The study consisted of face-to-face interviews and a review of their clinical and laboratory records. Data were analyzed using descriptive statistics and comparisons between groups made using Fisher’s test of exactness.
Results:
Eighty-seven teenagers were studied. The mean age was 15.5 ± 1.5 years. The majority (59%) were male. Ten study subjects (11.5%) were current smokers. A positive family history of asthma was found in 31% (n = 27) of the patients. All patients were on inhaled corticosteroid therapy. The majority of the study subjects (60.9%) had satisfactory asthma control as evidenced by a mean asthma control test score (ACT) of 19.2 ± 2.29. Fifteen patients (26.7%) had had an acute flare-up of the disease requiring hospitalization in the preceding 12 months, and 5.8% had had a previous intensive care unit admission.
Conclusion:
The majority of the teenagers studied had overall satisfactory asthma control, as determined by their ACT scores. The use of inhaled corticosteroids was standard, but the inhaler technique was largely unsatisfactory. The prevalence of tobacco use is a cause for concern.
Keywords
Profile
Teenage Asthmatics
Academic hospital
INTRODUCTION
Bronchial asthma is characteristically a diverse illness that results from chronically inflamed bronchi. The causes of asthma are not fully understood and probably involve an interaction between hereditary and environmental factors.[1] The inflammation within the airway wall is thought to be responsible for the bronchial hyperactivity of normal airway stimuli.[2] Data from Brazil indicate asthma to be highly prevalent among teenagers and lands a third of them in hospital annually.[3]Adolescents are a particularly vulnerable group to asthma, and both its prevalence and death rate are increased in this patient population in comparison to younger children.[3-5] The pattern of asthma may change majorly during adolescence.[6]
Adolescents tend to have a hard time managing a chronic illness such as asthma. They are known to be at a stage where there is a search for greater autonomy and self-care. They are changing socially and emotionally and noticing alterations in their relationships with friends and family.[7,8] It is during adolescence that limits are tested, unhelpful attitudes dominate, risky behavior is more acceptable, and experimentation with drugs such as tobacco is dominant.[9]
Asthma, such as other chronic illnesses, requires long-term compliance with therapy and monitoring on a regular basis. Adjustments to therapy are affected regularly, and being able to care for oneself is an integral part of asthma management. Adherence to asthma medication has been found to be especially low among children and teenagers.[10] Barriers to medication adherence, such as forgetfulness, have been reported to be more prevalent in this age group. Other teenage-specific barriers to medication adherence are pressure from peers, a sense of insecurity, or invincibility.[9]
For teenagers, the idea of managing a chronic medical condition or taking medication in front of peers carries with it a degree of awkwardness. Depression and anxiety are not unheard of in this patient population, and these psychological factors may result in poor asthma management.[11] Smoking and second-hand smoke can cause sudden and severe asthma flare-ups.[12]
Teenagers may prefer one inhaler device over the other despite being good and competent with their use.[13,14] In particular, many teenagers prescribed a metered dose inhaler with a spacer do not use the spacers as they are felt to be too bulky and inconvenient.[15]
Studies have, however, shown that with communication and commitment, asthmatic teens are able to take on more responsibility for control of their disease gradually.[16-18]
Aims and objectives
Aim
The aim of the study was to describe the control of asthma among teenage asthmatics attending the respiratory outpatient clinic of an academic center.
Objectives
The objectives of this study were as follows:
To assess their overall Asthma control using the asthma control test (ACT)
To assess inhaler technique using a standardized tool (inhaler device assessment tool)
To assess the teenage asthmatics’ knowledge of the names of their medication and how they are to be used.
MATERIALS AND METHODS
This study was a cross-sectional study of teenage asthmatics who attend the respiratory clinic at Dr. George Mukhari Academic Hospital, an academic hospital in Pretoria, South Africa. The study methods included face-to-face interviews and a review of the hospital and laboratory records of the patients meeting the inclusion criteria and willing to participate. The study was approved by the Institutional Review Board (SMUREC) before commencement (SMUREC/M/315/2022:PG). Informed consent was obtained from those 18 years or older, and an assent form was signed by those participants under the age of 18, with their parents signing the informed consent form, after the researcher had explained the study purpose and format. Patients who were unable to or unwilling to sign the consent or/assent forms were excluded from the study.
The following information was obtained from the interview and record review: Demographic data, approximate age at asthma diagnosis, medication currently being taken and how each medicine was taken, current symptom control as determined by ACT, current spirometry, and inhaler technique. The inhaler technique was assessed as being good only if the patient scored 5 on the inhaler device assessment tool.
The ACT is a short symptom-based patient tool questionnaire used to monitor asthma control.[19,20] It consists of five questions with a rating of 1–5, and patients are asked to recall their symptoms and daily functioning in the past 1 month. The ACT assesses the frequency of shortness of breath, the use of rescue medication, the effect of asthma on daily functioning, and the overall assessment of asthma control. The score ranges from 5 to 25, with 5 being poorly controlled and 25 being very well controlled. A score of >19 indicates adequate control.
Sample size calculation
Sample size calculation was based on a recent study conducted in Cape Town, which suggested that the prevalence of asthma among adolescents was 34.5%.[21] We assumed that this would be the prevalence level among adolescent patients managed at our academic institution. With this assumption in mind, the calculated sample size for this study was 87 adolescents. The study sample size was calculated to ensure 80% power of the study and a two-sided alpha error limit of 0.05.
Data analysis
The study data were analyzed following transcription into Microsoft Excel using the Statistical Package for the Social Sciences (IBM, Inc. USA; Version 27.0). Descriptive statistical analyses, such as range, mean, and standard deviation, were calculated for data with a normal distribution, and median estimation of data was applied to variables that did not conform to a normal distribution. The clinical profiles of the teenage patients with asthma were described as proportions and percentages with appropriate 95% confidence interval values.
Comparisons between groups were made using Fisher’s test of exactness. Differences found from such analyses were emphasized if P < 0.05.
RESULTS
Demographic data
A total of 87 teenagers with asthma were studied. The mean age of the group was 15.4 ± 1.5 years. Figure 1 demonstrates the age distribution of the cohort. Fifty-two (59.8%) of the patients identified with the male gender.
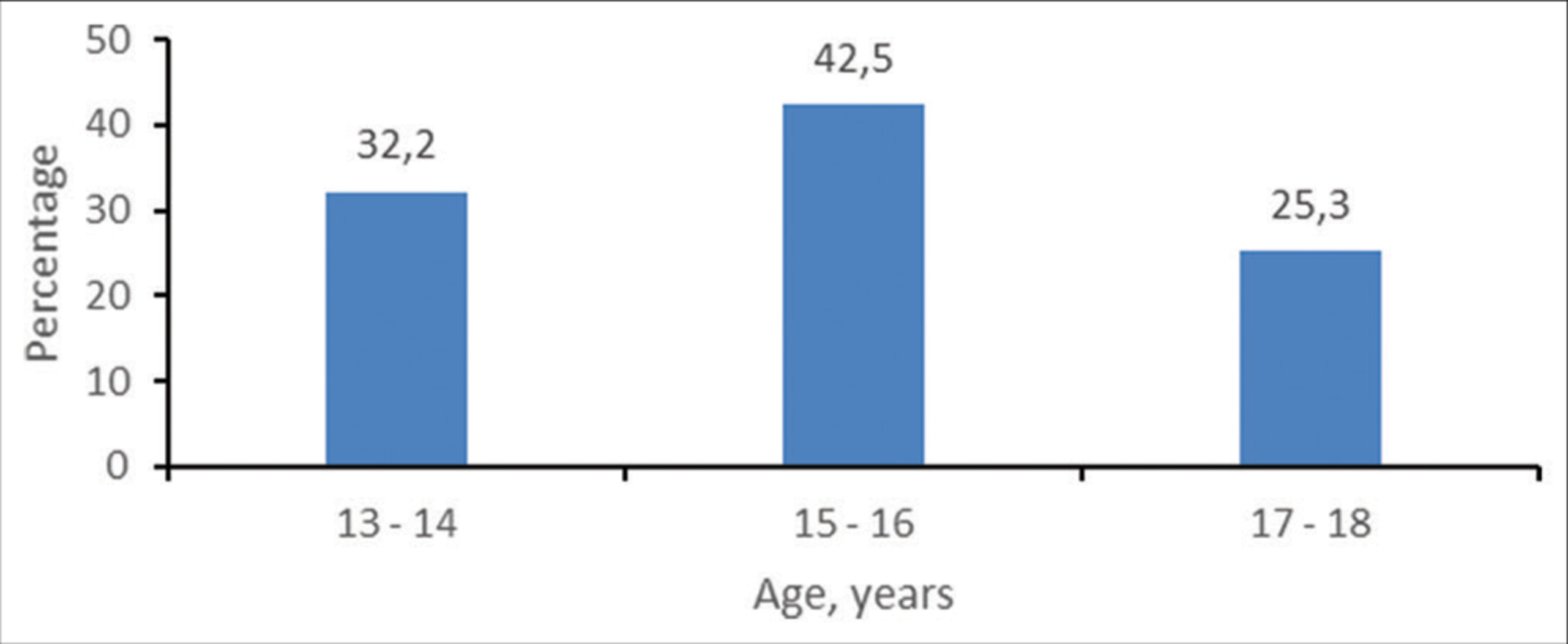
- Age distribution of the cohort.
The school grade and number of learners in each are presented in Table 1. The majority (25.3%) were in grade 11.
| Grade | Frequency (%) |
|---|---|
| 5 | 2 (2.3) |
| 7 | 1 (1.1) |
| 8 | 16 (18.4) |
| 9 | 18 (20.7) |
| 10 | 18 (20.7) |
| 11 | 22 (25.3) |
| 12 | 10 (11.5) |
| Total | 87 (100) |
Forty-one patients (47.1%) were diagnosed with asthma between the ages of 3 and 5. Table 2 summarizes the cohort’s ages at diagnosis.
| Age, years | Frequency (%) |
|---|---|
| 3–5 | 41 (47.1) |
| 6–7 | 25 (28.7) |
| 8–9 | 10 (11.5) |
| 10–11 | 8 (9.2) |
| 12–13 | 3 (3.5) |
| Total | 87 (100) |
Twenty-seven patients (31%) had a positive family history of Asthma. Table 3 is the breakdown of affected family members.
| History of asthma | Frequency (%) |
|---|---|
| No | 60 (69.0) |
| Father | 11 (12.7) |
| Mother | 6 (6.9) |
| Grandparent | 4 (4.6) |
| Sister | 3 (3.4) |
| Brother | 3 (3.4) |
| Total | 87 (100) |
The percentages of males and females with a family history of asthma (28.8% vs. 34.3%) did not differ significantly (P = 0.641).
The percentage of patients aged 5 years or younger at diagnosis and the percentage of patients older than 5 years at diagnosis with a family history of asthma (26.8% vs. 34.8%) did not differ significantly (P = 0.491).
Ten (11.5%) teenagers were current tobacco users. The percentages of males and females that smoked (11.5% vs. 11.4%) did not differ significantly (P = 1.000).
Forty-seven (54%) of the teenagers with asthma did not take part in any form of sporting activity. Twenty (42.5%) of them indicated that they were fearful of aggravating their disease. The remainder had no tangible reasons for not participating in a sporting activity.
Several of the patients had comorbidities usually associated with asthma, including eczema, allergic rhinitis, and conjunctivitis. The distribution and frequency of these are summarized in Table 4.
| Comorbidity | Frequency (%) |
|---|---|
| None | 25 (28.7) |
| Rhinitis+Conjunctivitis | 32 (36.8) |
| Rhinitis | 15 (17.2) |
| Rhinitis+Conjunctivitis+Eczema | 8 (9.2) |
| Rhinitis+Eczema | 4 (4.6) |
| Conjunctivitis | 1 (1.2) |
| Nasal polyps | 2 (2.3) |
| Total | 87 (100) |
All patients had spirometry performed, and 17 (19.5%) had abnormalities in keeping with an obstructive ventilatory defect.
Knowledge of the names of their asthma medication and appropriateness of use (how often and when) was deemed satisfactory in 67.8% (n = 69 ) of the patients. Inhaler techniques were assessed as good in only 51.7% (n = 45) of the study subjects.
Twenty-one (24.1%) subjects were provided with a spacer, 18 (20.7%) had a home nebulizer, and 93.1% (n = 81) had an asthma action plan.
All patients were on an inhaled steroid. The summary of the treatment received by this cohort is in Table 5.
| Treatment | Frequency (%) |
|---|---|
| ICS, SABA, Ns | 33 (37.9) |
| ICS, SABA | 26 (30.0) |
| ICS, SABA, Ns, Ocular steroids | 16 (18.4) |
| ICS, SABA, Theophylline | 4 (4.6) |
| ICS, SABA, Montelukast | 3 (3.4) |
| ICS, SABA, Ns, Theophylline | 3 (3.4) |
| ICS, SABA, Montelukast, Ns | 2 (2.3) |
| Total | 87 (100) |
Ns: Nasal steroids, SABA: Short-acting beta2 agonist, ICS: Inhaled corticosteroids
Asthma control
Many of the study subjects had satisfactory scores on the ACT [Figure 2].
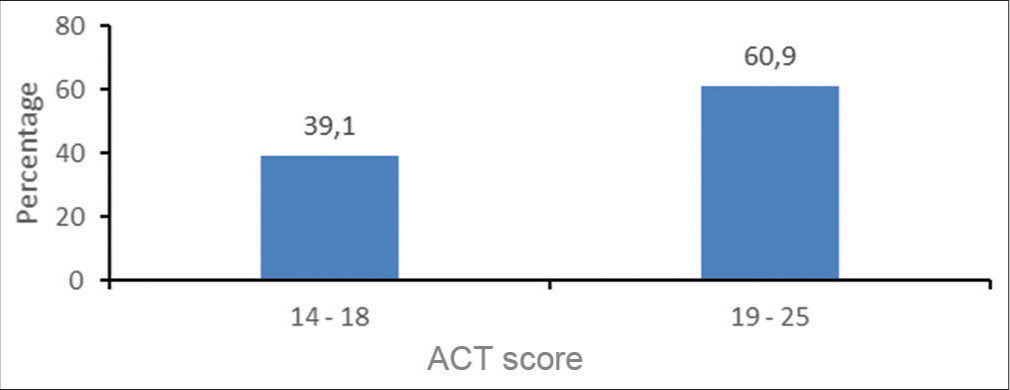
- Asthma control test scores (ACT).
The percentages of males and females in categories 14– 18 (42.3% vs. 34.3%) did not differ significantly (P = 0.507). The same conclusion was applicable to category 19–25, namely, that the percentages of males and females in category 19–25 (57.7% vs. 65.7%) did not differ significantly (P = 0.507).
Fifteen (26.7%) of the subjects had had at least one asthma attack requiring hospitalization within the preceding 12 months. One patient had suffered two exacerbations. Five patients (5.8%) had a previous history of admission to the intensive care unit (ICU) for an asthma flare-up.
Among the patients who had good inhaler techniques, 3 (6.7%) were admitted to ICU, and among patients with poor inhaler techniques, 2 (4.8%) were admitted to ICU. The two percentages did not differ significantly (P = 1.000).
Five of the patients (8.5%) whose knowledge of asthma therapy was deemed satisfactory were admitted to ICU.
DISCUSSION
Gender differences
The majority (59.8%) of the study subjects were male. Childhood asthma and the severity of symptoms have been described as more prevalent among males than females.[22,23] During adolescence, however, the trend has been observed to change, with the prevalence and symptoms more severe in females than in males. Some of the suggested explanations for this switch have included increased hormonal changes and changes in gender-specific differences and environmental exposure.[24]
Family history
Thirty-one percent of the teenagers in this study had one or more family members with a firm diagnosis of bronchial asthma. A family history of asthma in one or more first-degree relatives has consistently been identified as a risk factor for asthma, with maternal asthma history having a much stronger association.[25-27] A systematic review of ten studies looking at family history as a predictor of asthma risk found sensitivity to range from 4% to 43%, a positive predictive value ranging from 11% to 37%, and a negative predictive value of 86–97%.[25]
A study conducted by a group of researchers at the University of Michigan in the United States of America showed a strong hereditary pattern in developing asthma from a biological parent or grandparent. The study was conducted among children and adolescents between the ages of 5–19 years of age. The main findings of the study were that children with a parent with asthma were about twice as likely to have asthma, and those with a parent and grandparent with asthma were 4 times more likely to have asthma, regardless of gender, ethnic background, and birth order.[28]
Concomitant use of tobacco
Tobacco was routinely used by 11.5% of the study population. A study carried out among the youth in Virginia in the USA in 2014 to assess smoking and asthma status among different high school students showed that around 16.9% of the asthmatic adolescence used tobacco.[29] The previous studies have suggested that adolescents with asthma are more likely to use tobacco more regularly than their non-smoking counterparts.[30-32] The explanation for this remains unclear as a comparable association with risk factors for smoking between asthmatics and non-asthmatics was found.[32] According to the Centers for Disease Control and Prevention in 2016, the use of different types of tobacco products in Florida in the USA was higher among middle and high school students with asthma than among those without asthma. Among students with asthma, more than 10% (11.1%) of middle school and more than a quarter (27.9%) of high school students reported current use of some form of tobacco. The use of tobacco products among non-asthmatics in this cohort was 7.9% and 24.2%, respectively.[32]
It has been accepted for many years that asthma and active cigarette smoking interact to the extent that asthma symptoms become more severe, the decline in lung function is accelerated, short-term therapeutic response to corticosteroids is impaired, and rates of hospitalization increase.[33,34]
Cigarette smoking may modify inflammation that is associated with asthma. Although the mechanisms of corticosteroid resistance in asthmatic smokers are not fully elucidated, there is a suggestion that it is the result of the changes in the airway cellular milieu and changes in the glucocorticoid receptor ratios.[33,34]
Inhaler technique
Just over 50% (51.7%) of the candidates in this study were deemed to have good inhaler technique. A study conducted among 140 adult asthmatics in Lagos, Nigeria found that only 22.1% and 37.3% showed the correct technique for the use of a metered dose inhaler and dry powder devices, respectively.[35]
Studies have suggested that asthmatics with poor inhaler technique tend to have suboptimal control and have a heightened risk of flare-ups.[36,37]
Asthma medication knowledge
Knowledge of asthma therapies was deemed unsatisfactory in this cohort, with only 67.8% (n = 59) of the participants familiar with the names, appropriate times of use, and dosages of their medication. This was a little better than the results of a study conducted in Rochester in the USA where, of the 176 participants, only 31% could successfully identify and name their treatment.[38]
An Australian study looking at patient knowledge of asthma treatment found that most (83%) could describe the actions of preventer and 92% reliever medication appropriately.[39] This was different from research carried out in Trinidad a few years earlier, where only 53% of their chest clinic patients understood the rationale for using preventer and reliever medications.[40]
Better understanding of therapy is vital for asthma management as asthmatics are likely to be using multiple medication and delivery devices.[39] Adolescence with a satisfactory understanding of their therapy had better treatment adherence, less acute health care, and exposure to in-school directly observed therapy.[41]
Overall asthma control
Fifty-three of the study subjects (60.9%) in this cohort had satisfactory asthma control, as evidenced by a score of between 19 and 25 on the ACT. A previous study of 123 subjects in South African inland provinces showed that out of the 90 study participants who claimed that their asthma was controlled, only 25 (27.8%) were correctly classified as “controlled” according to the ACT.[42]
A French study looking at asthma control among adolescents with severe asthma transitioning to specialist care found that nearly three-quarters (73%) of patients had well controlled asthma, with an ACT scores ≥20 during transition.[43]
According to a study by Tosca et al., despite the widespread dissemination of asthma guidelines, only half of the adolescents have well-controlled asthma,[44] and data from the Global Asthma Network Phase I cross-sectional epidemiological study (2015–2020) suggest that asthma was well-controlled in only 55.4% of adolescents.[45]
Hospitalization rate
Five percentages of our participants required hospital admission in the past 1 year. A study in Brazil in 2021 that looked at 322 teenagers with asthma ranging from mild, moderate to severe persistent asthma found that 7% of the participants had had a hospital admission for a flare-up of asthma in the previous 12 months.[46] A recent Brazilian study suggested a tendency to reduced hospitalization for children and adolescents with asthma.[47]
Outside of these two studies, we could not find any other studies that focused on the hospitalization rates of teenage asthmatics.
ICU admissions
The ICU admission rate in this study was 5.1%. Studies looking specifically at rates of adolescent asthmatics admissions to ICUs were hard to come by. A study done in 2000 by Pendergraft et al. looked at ICU admissions in 215 hospitals, and found that 10.1% of the admissions were a result of severe asthma and 2.1% of the patients needed mechanical intubation and ventilation.[48]
Study strengths
The data collection sheet was piloted on ten patients and peer reviewed in our department to ensure that it collected the data of interest, and adjustments were made where necessary. The sample size was statistically derived to ensure reliability of the results. The ACT test is a standardized and worldwide test for asthma control. The researcher double-checked entries into the data collection to ensure accuracy. Participants were given adequate time to answer questions and lead questions were avoided.
Study limitations
This was a single center study and the results may not be generalizable. Some of the information collected was subject to recall and recall bias might have compromised the data.
Recommendations
The study did not focus on adherence to asthma therapy. This is an important component of its management and it would be helpful to evaluate adherence; however, it has to be acknowledged that is not easy to assess adherence given the limited accepted methods of assessing compliance.
CONCLUSION
Overall, the control of asthma among teens transitioning to adult care services in our hospital appears satisfactory. There is room for improvement in the following aspects of their care: Inhaler technique and information on asthma pharmacotherapy. A lot needs to be done to dissuade teenagers with asthma from taking up the use of tobacco products. Special consideration should be given to assessing and managing teenagers with asthma. The beliefs and fears of teenagers about their asthma and its treatment need to be acknowledged and addressed by family members and healthcare workers.
Ethical approval
The research/study approved by the Institutional Review Board at Sefako Makgatho Health Sciences University, number (SMUREC/M/315/2022:PG), dated October 22, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Advances and recent developments in asthma in 2020. Allergy. 2020;75:3124-46.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of airway hyper responsiveness. J Allergy Clin Immunol. 2006;118:551-9.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma in children and adolescents in Brazil: Contribution of the international study of asthma and allergies in childhood (ISAAC) Rev Paul Pediatr. 2014;32:114-25.
- [CrossRef] [PubMed] [Google Scholar]
- Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123:S131-45.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in childhood asthma: Prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315-22.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5:224-34.
- [CrossRef] [PubMed] [Google Scholar]
- Keeping pace with adolescent asthma: A practical approach to optimizing care. Pulm Ther. 2022;8:123-37.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of asthma in adolescents. Clin Respir J. 2009;3:69-76.
- [CrossRef] [PubMed] [Google Scholar]
- Transition for adolescents and young adults with asthma. Front Pediatr. 2019;7:301.
- [CrossRef] [PubMed] [Google Scholar]
- Medication adherence in the asthmatic child and adolescent. Curr Allergy Asthma Rep. 2011;11:454-64.
- [CrossRef] [PubMed] [Google Scholar]
- Management issues for adolescents with cystic fibrosis. Pulm Med. 2012;2012:134132.
- [CrossRef] [PubMed] [Google Scholar]
- Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28:323-33.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment adherence in adolescents with asthma. J Asthma Allergy. 2020;13:39-49.
- [CrossRef] [PubMed] [Google Scholar]
- What do adolescents with asthma really think about adherence to inhalers? Insights from a qualitative analysis of a UK online forum. BMJ Open. 2017;7:e015245.
- [CrossRef] [PubMed] [Google Scholar]
- Health experiences of adolescents with uncontrolled severe asthma. Arch Dis Child. 2010;95:985-91.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring factors influencing asthma control and asthma-specific health-related quality of life among children. Respir Res. 2013;14:26.
- [CrossRef] [PubMed] [Google Scholar]
- The adolescent with asthma. Paediatr Respir Rev. 2014;15:146-53.
- [CrossRef] [PubMed] [Google Scholar]
- Adolescence and asthma management: The perspective of adolescents receiving primary health care. Rev Paul Pediatr. 2014;32:171-6.
- [CrossRef] [Google Scholar]
- Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59-65.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549-56.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma in South African adolescents: A time trend and risk factor analysis over two decades. ERJ Open Res. 2021;7:576-2020.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17:19.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of gender on asthma in childhood and adolescence: A GA2LEN review. Allergy. 2008;63:47-57.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of family history in asthma during the first 27 years of life. Am J Res Crit Care Med. 2013;188:521-628.
- [CrossRef] [PubMed] [Google Scholar]
- Risk for asthma in offspring of asthmatic mothers versus fathers: A meta-analysis. PLoS One. 2010;5:e10134.
- [CrossRef] [PubMed] [Google Scholar]
- Family history as a predictor of asthma risk. Am J Prev Med. 2003;24:160-9.
- [CrossRef] [PubMed] [Google Scholar]
- Examining the association between childhood asthma and parent and grandparent asthma status: Implications for practice. Clin Pediatr (Phila). 2010;49:535-41.
- [CrossRef] [PubMed] [Google Scholar]
- Association between high school students' cigarette smoking, asthma and related beliefs: A population-based study. BMC Public Health. 2016;16:913.
- [CrossRef] [PubMed] [Google Scholar]
- Adolescent tobacco smoke exposure, respiratory symptoms, and emergency department use. Pediatrics. 2018;142:e20180266.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma and cigarette smoking: A review of the empirical literature. J Asthma. 2010;47:345-61.
- [CrossRef] [PubMed] [Google Scholar]
- Smoking in young people with asthma. J Public Health (Oxf). 2007;29:343-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of inhaler techniques among asthma patients seen in Nigeria: an observational cross sectional study. Ann Med Health Sci Res. 2014;4:67-73.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of inhaler technique on clinical outcomes in adolescents and adults with asthma: A systematic review. Respir Med. 2022;202:106949.
- [CrossRef] [PubMed] [Google Scholar]
- Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy Asthma Clin Immunol. 2013;9:8.
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge of inhaled therapy and responsibility for asthma management among young teens with uncontrolled persistent asthma. Acad Pediatr. 2018;18:317-23.
- [CrossRef] [PubMed] [Google Scholar]
- Patient medication knowledge and adherence to asthma pharmacotherapy: A pilot study in rural Australia. Ther Clin Risk Manag. 2005;1:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding and use of inhaler medication by asthmatics in specialty care in Trinidad: A study following development of Caribbean guidelines for asthma management and prevention. Chest. 2002;121:1833-40.
- [CrossRef] [PubMed] [Google Scholar]
- Adolescent knowledge of when to use inhaled asthma medications: Implications for management. J Adolesc Health. 2023;72:623-8.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma control among adolescents in the inner provinces of South Africa: Perception v. reality. S Afr J Child Health. 2023;17:1-3.
- [CrossRef] [Google Scholar]
- Maintenance of asthma control in adolescents with severe asthma after transitioning to a specialist adult centre: A French cohort experience. J Asthma Allergy. 2022;15:327-40.
- [CrossRef] [PubMed] [Google Scholar]
- The real-world “ControL'Asma” study: A nationwide taskforce on asthma control in children and adolescents. Allergol Immunopathol (Madr). 2021;49:32-9.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma management and control in children, adolescents, and adults in 25 countries: A Global asthma network phase I cross-sectional study. Lancet Glob Health. 2023;11:e218-28.
- [CrossRef] [Google Scholar]
- Hospital admission rate in children and adolescents with mild persistent asthma. Pediatr Pulmonol. 2021;56:1889-95.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the trend of hospitalizations for asthma in children and adolescents in Brazil. J Pediatr (Rio J). 2021;97:309-14.
- [CrossRef] [PubMed] [Google Scholar]
- Rates and characteristics of intensive care unit admissions and intubations among asthma-related hospitalizations. Ann Allergy Asthma Immunol. 2004;93:29-35.
- [CrossRef] [PubMed] [Google Scholar]






