Translate this page into:
Advancing pediatric lung health in Africa: COVID-19 and beyond
*Corresponding author: Marco Zampoli, Division of Paediatric Pulmonology, Red Cross War Memorial Children’s Hospital, University of Cape Town, Cape Town, South Africa. m.zampoli@uct.ac.za
-
Received: ,
Accepted: ,
How to cite this article: Zampoli M, Gray D, Lake L, Levin M, Vanker A, Zar HJ. Advancing pediatric lung health in Africa: COVID-19 and beyond. J Pan Afr Thorac Soc 2020;1(1):6-14.
Abstract
Respiratory illnesses in children are an important cause of morbidity and mortality in Africa where poverty, food insecurity, malnutrition, and human immunodeficiency virus infection are aggravating factors in many countries. These factors, in addition to under resourced health-care infrastructure, remain important barriers to improving child lung health outcomes in Africa. However, despite these challenges, there have been significant recent advancements in understanding the epidemiology, causes, measurement tools, and treatment of childhood respiratory illnesses. In this review, we highlight some advances in childhood pneumonia, tuberculosis, asthma, and other important non-communicable lung diseases common in children. Furthermore, we discuss the role of environmental influences on children’s lung health in Africa and highlight novel methods of measuring lung function in children. Although children contribute a small role in the global epidemiology of COVID-19 pandemic, socioeconomic and health-care delivery disruptions caused by government responses to contain the pandemic have resulted in significant indirect setbacks to child health. We further highlight how the COVID-19 pandemic has impacted areas of pediatric lung health and discuss ways to mitigate effects of COVID-19 in Africa.
Keywords
Pediatrics
Lung health
Africa
COVID-19 pandemic
INTRODUCTION
Childhood respiratory illnesses contribute significantly to under 5-year mortality and morbidity in lower-middle-income counties (LMICs) across the world. Despite declining mortality and morbidity associated with childhood pneumonia during the Millennium Development Goal period 2000–2015, 0.9 million children younger than 5 years died of pneumonia in 2015, with half of these deaths occurring in Africa and Southeast Asia.[1] While reducing childhood pneumonia, mortality is an important global priority, reducing morbidity and mortality from other respiratory illnesses in children requires equal attention. Clear evidence exists of the importance of early-life origins of chronic obstructive pulmonary disease (COPD) in adults. Although smoking and exposure to biomass fuels in poor countries are important causes of COPD, early-life factors including genetic predispositions, prenatal exposure to cigarette smoke, prematurity, lower respiratory infections, childhood asthma, and air pollution all contribute to diminishing maximally attained lung function and rate of lung function decline in later life.[2] Targeted interventions in early childhood that reduce the burden of respiratory diseases in children are key to improving lung health for all populations. There have been numerous advances in prevention, diagnosis, monitoring, and treatment of childhood respiratory diseases in Africa. However, many of these achievements have been setback and potentially reversed due to indirect harmful consequences of the COVID-19 pandemic on child health, especially in LMIC where diversion of limited resources away from child health interventions is unavoidable.[3] Key strategies to protect children in LMIC from COVID-19 have been proposed.[4] In this review, we highlight important advances in the epidemiology, diagnosis, and treatment of important childhood respiratory illnesses in Africa and other LMIC. Furthermore, we discuss the potential impact and consequences of COVID-19 on these advances and suggest strategies to mitigate the effects.
NEW ADVANCES IN CHILDHOOD PNEUMONIA AND TUBERCULOSIS (TB)
The incidence and severity of childhood pneumonia has reduced substantially in the past 2 decades [Figure 1], with improvements in living conditions, strengthened strategies to prevent or manage pediatric human immunodeficiency virus (HIV) and improved immunization strategies, particularly pneumococcal conjugate vaccine and Haemophilus influenzae type b conjugate vaccine (HiB). However, pneumonia remains the commonest cause of death in children under 5 years outside the neonatal period, with almost 800,000 deaths in 2018, with the burden skewed toward LMICs, especially Africa.[1,5] Further, pneumonia may impair long-term health, leading to impairments in lung function, and setting children on a trajectory for the development of chronic respiratory disease through the life course.[6]
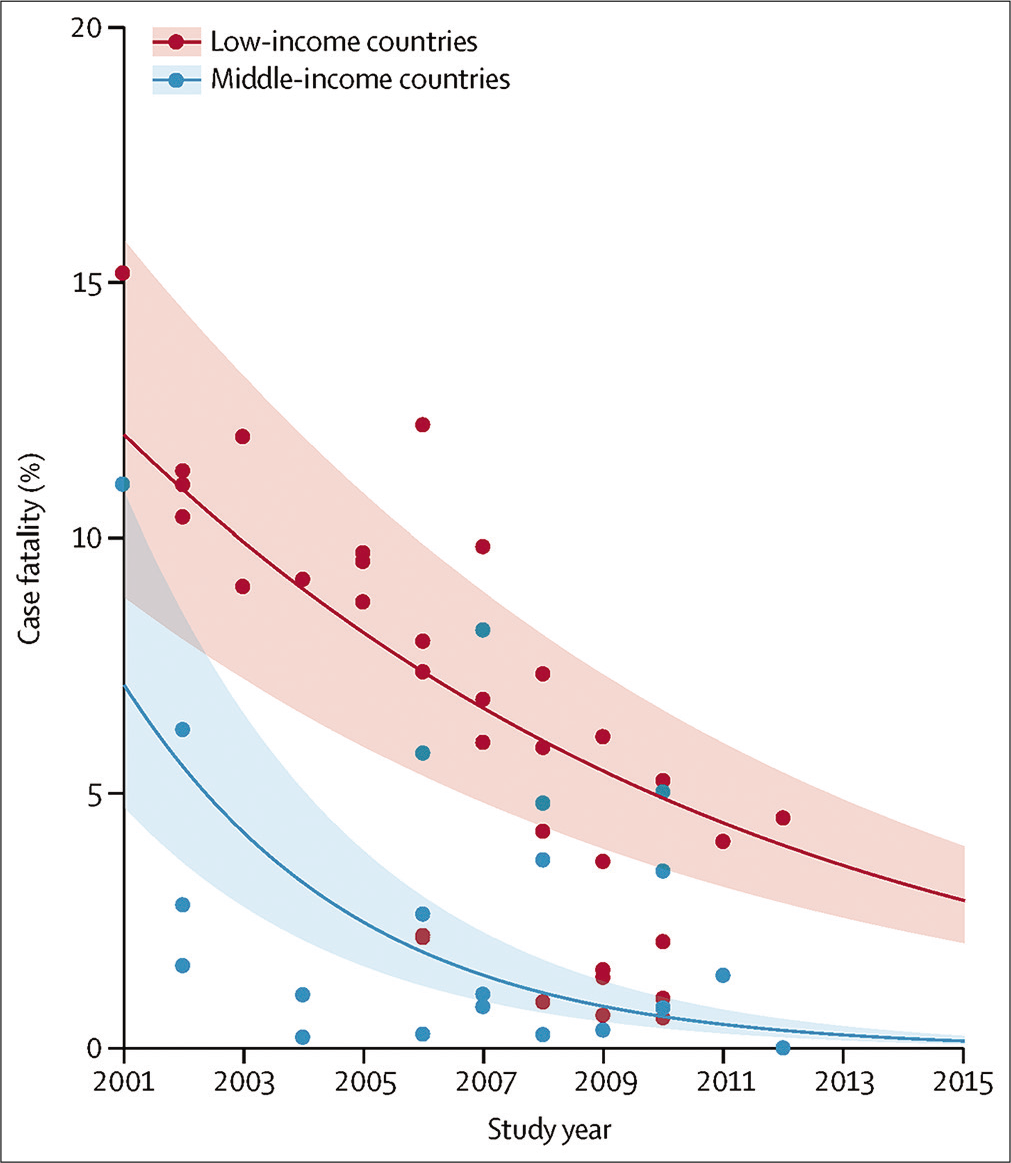
- Declining in-hospital case fatality rate for young children admitted to hospital with pneumonia in low-income and middle-income countries between 2001 and 2015; shaded bands show 95% uncertainty intervals reproduced from ref 1: McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: A systematic analysis. Lancet Glob Health 2019;7:e47-57.
Advances in preventive interventions, coupled with improved diagnostic methods and better sampling strategies, have enabled more accurate diagnosis of the pathogenesis and etiology of childhood pneumonia. With strengthened immunization strategies, and reduction in numbers of HIV-infected children and more widespread use of ART, there has been a change in the relative contribution of different pathogens in the pathogenesis of pneumonia. Pneumonia usually results from interaction of several organisms, with multiple organisms cooccurring.[7,8] Viral pathogens are common, with respiratory syncytial virus, the most common viral pathogen. Non-typeable H. influenzae and Staphylococcus aureus are now important bacterial pathogens. Mycobacterium tuberculosis is common in the context of acute pneumonia in African children.[9] Children with severe pneumonia, or those with ambulatory pneumonia who are malnourished, HIV infected or have a household TB contact should be carefully evaluated for TB. Advances in TB diagnosis in children have indicated that microbiological diagnosis is feasible and useful even in young children.[10] Better sampling methods, predominantly induced sputum collection coupled with rapid PCR testing for M. tuberculosis and for rifampicin resistance (Xpert and more recently Xpert Ultra which has higher sensitivity than Xpert), are useful for microbiological confirmation and guiding treatment.[10] There are growing concerns that disruptions to primary care TB programs and intervention caused by COVID-19 lockdown interventions may lead to increased incidence of childhood TB.
Pneumonia deaths can be prevented with the available preventive and management interventions. Prevention involves mitigating some of the key risk factors for pneumonia or for severe disease. Maternal factors include maternal education, avoidance of smoking through pregnancy onwards, good antenatal care with optimization of maternal physical and mental health, well-controlled HIV through use of ART in mothers living with HIV, and breastfeeding. Child factors include good nutrition, immunization and early diagnosis of HIV, and use of ART and cotrimoxazole prophylaxis in children living with HIV. Environmental factors include adequate living and socioeconomic conditions, and no or minimal exposure to tobacco smoke or pollution. Health system factors include timely access to effective preventive (such as immunization) or management strategies. Access to a health facility with timely administration of antibiotics and oxygen remains a challenge in areas of Africa, with many childhood pneumonia deaths still occurring outside a health facility.
There is clear epidemiological evidence that SARS-CoV-2 infection transmission and COVID-19 are less significant in children compared to adults. Globally, children represent 1–2% of SARS-CoV-2 cases, the majority reported to have mild symptoms or asymptomatic.[11] Severe pneumonia due to SARS-CoV-2 is rare and usually associated with significant underlying medical conditions. Interestingly, seroprevalence studies reported higher (6.7–9.7%) prevalence of infections in children over 5 years age.[11] Coinfection with other respiratory viruses and Mycoplasma pneumoniae has been reported in 40–50% of cases, confirms the polymicrobial nature of childhood pneumonia.[11] Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection has emerged as potentially serious COVID-19 manifestation in children worldwide, including in South Africa.[12] Although rare, children with MIS-C are at increased risk of cardiac dysfunction and critical illness. A high index of suspicion and awareness of this condition in children is thus needed as the COVID-19 pandemic spreads throughout Africa.
ASTHMA
Asthma is a common chronic disease in children in many African countries. The prevalence of childhood asthma in Africa is higher than the global average and the burden of childhood asthma in Africa is increasing.[13] Asthma is less common in children living in rural areas than those living in large urban areas. Multiple protective factors may be operable in rural environments.[14] Conversely, risk factors unique to the urban environment may be pro-inflammatory. The assessment and diagnosis of asthma in childhood remains clinical but can be challenging in young children and in the presence of comorbidities. Management of asthma includes the same four basic principles of management of other allergic conditions namely: Patient education, trigger avoidance, pharmacotherapy, and immunotherapy. Asthma therapy aims to achieve as normal a quality of life, free of symptoms, and limitations.
The Global Initiative for Asthma (GINA) treatment guidelines underwent fundamental changes in 2019 due to concern of increased risk of morbidity and death with the previous long-standing approach of commencing mild asthma treatment with short-acting β2-agonists (SABAs) alone.[15] New guidelines do not recommend asthma treatment with a reliever medication alone. Even children in the former category of “intermittent asthma” should be prescribed an inhaled corticosteroid with reliever medication if required.
New GINA Guidelines
New GINA guidelines[16] recommend the following stepwise approach as the preferred and alternative controller therapy in adults and children above the age of 12 years. In children 5–11 years, recommended first line controller medication is low does ICS with intermittent SABA as reliever or intermittent ICS/LABA combination as controller and reliever. For reliever therapy GINA recommends using a low dose ICS/formoterol combination, or alternatively SABA as needed
Step 1: Low dose inhaled corticosteroids (ICS)/formoterol combination, or ICS given whenever SABA given
Step 2: Daily low dose ICS or as needed ICS/formoterol; alternatives oral leukotriene receptor antagonists (LTRA) or low dose ICS taken with long acting beta 2 agonists (LABA)
Step 3: Regular low dose ICS/LABA; alternatives medium dose ICS, low dose ICS + LTRA
Step 4: Medium dose ICS/LABA; alternatives high dose steroids, add on tiotropium, add on LTRA
Step 5: High dose ICS/LABA and refer.
Access to spacer devices in Africa and achieving adequate inhaled drug deposition in children remains a challenge. However, low-cost spacer devices can be constructed out of a plastic bottles which have been shown to be as effective as commercial devices.[17] A hole large enough to take the mouthpiece of the multidose inhaler (MDI) is cut (or burned) in the end of the bottle to form a simple low-cost non-valve spacer [Figure 2].
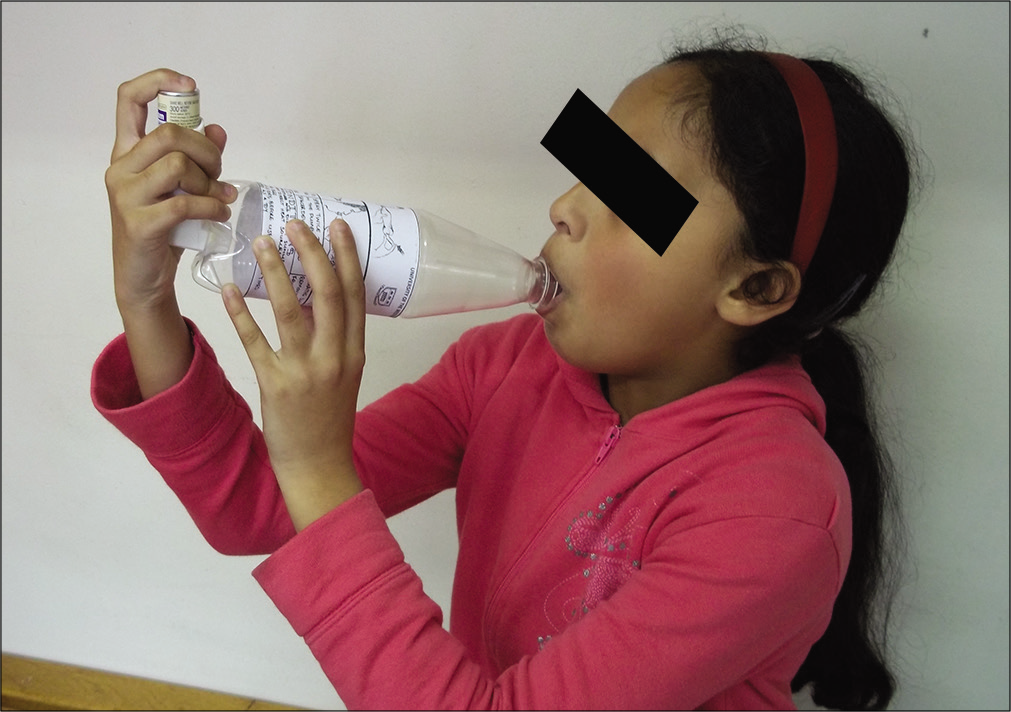
- Low-cost spacer device made from a plastic bottle.
Treatment of acute asthma during COVID-19 pandemic
Inhaled SABAs are essential for treating acute asthma. However, due to increased risk of aerosolizing respiratory droplets in SARS-CoV-2-infected patients, amended acute asthma guidelines have been published for settings with high COVID-19 prevalence. Patients should be stratified into three categories: Mild-moderate, severe, and life threatening, with nebulized oxygen-driven therapy restricted to those with life-threatening asthma only and with the use of personal safety equipment.[16] In addition, systemic bronchodilator therapy with intravenous magnesium sulfate should be considered earlier in treating severe asthma and replacing nebulizer delivery of SABA with multidosing MDI therapy using a spacer in those with life-threatening exacerbations.[16]
Primary care for asthma
International and national guidelines for childhood asthma have been produced, but implementation remains challenging. Challenges to implementation include lack of medication availability in primary care, lack of human resources, especially competent nursing staff, the limited scope of practice, and prescription of medication, for example, professional nurses at clinics, lack of access to care in certain settings, inadequate undergraduate training in allergology, and deficiencies in leadership and health management. Some of these deficiencies can be addressed by strengthening allergy services at primary health care, training community health workers, nurses and doctors, and making suitable protocols available. Programmatic aspects include the provision of necessary infrastructure, equipment and medication, improving communication and referral between different levels of care, and improving undergraduate training in allergy and immunology.
OTHER CHRONIC RESPIRATORY CONDITIONS
The prevalence and burden of other non-communicable respiratory illnesses affecting children is poorly documented and likely to be underestimated due to overwhelming incidence of acute respiratory infections (ARIs) including TB. HIV-associated chronic lung diseases and bronchiectasis caused by recurrent lower respiratory tract infections (LRTIs) are common in HIV-infected children and cause significant morbidity and disability. Lower lung function associated with fixed airway obstruction, bronchiolitis obliterans, and bronchiectasis is the most common pathology described HIV-infected children.[18] Furthermore, lung function impairment persists in children and adolescents despite antiretroviral therapy.[19] Other chronic respiratory disorders including post-infectious bronchiolitis obliterans [Figure 3], pulmonary fibrosis, interstitial lung diseases, cystic fibrosis, sleep disordered breathing, and respiratory conditions associated with genetic disorders are underreported and probably overlooked in many African settings. The negative impact on COVID-19 on diagnosis and care and management of children living with such conditions is likely to be significant.
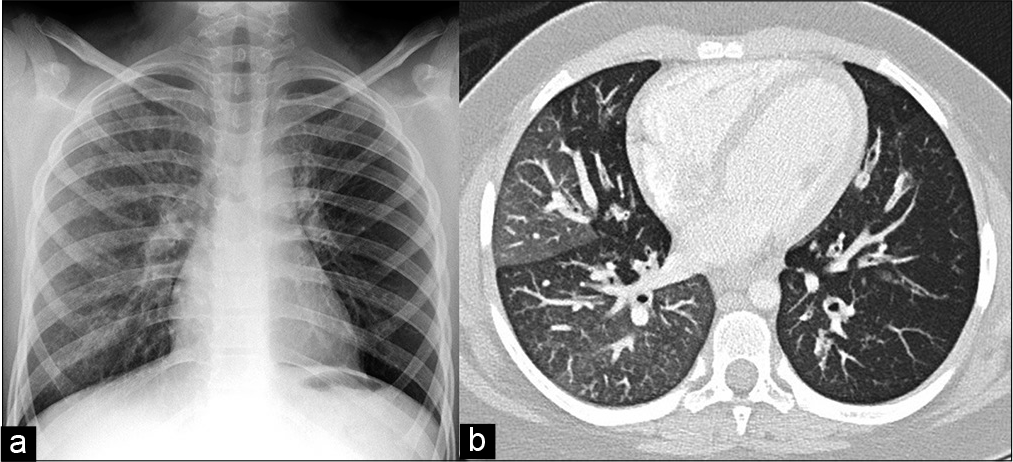
- Chest radiograph (a) and axial CT scan images (b) of chronic obstructive airway disease in a 10-year-old girl with Down syndrome: Hyperlucent left lung on chest radiograph indicates hypoperfusion and air trapping of the left lung. Mosaic attenuation pattern of lung fields and thickened, dilated bronchi are typical CT signs of post-infectious bronchiolitis obliterans with bronchiectasis.
Useful resources for asthma
The Allergy Foundation of South Africa (AFSA): Established to create awareness, to advise and educate patients, to promote responsible care, to assist with advocacy and to raise funds for professional development. The organisation runs a variety of educational programs aimed at patients and has an excellent website www.allergyfoundation.co.za with patient information leaflets, and an active Facebook page. AFSA provides education for health care professionals through online courses and interactive workshops for doctors, nurses, pharmacists and dieticians.
The Allergy in Africa Network and Webinar series spreads information to interested health care professionals in Africa. More information about these initiatives can be obtained by emailing training@allergyfoundation.co.za.
ENVIRONMENTAL EXPOSURES AND PEDIATRIC LUNG HEALTH
Environmental exposures, especially air pollution, are increasingly recognized as impacting negatively on health and disproportionately affecting LMIC.[20] In Africa, more than 90% of children are exposed to air pollution levels above acceptable ambient standards.[21] While LRTIs remain the leading cause of under-5 morbidity and mortality, the burden of pediatric non-communicable respiratory diseases including asthma is significant, both in Africa and globally.[1,22,23] Systematic reviews have shown that exposure to indoor air pollution almost doubles the risk of pneumonia in young children,[24-26] and studies from Africa are similar. A Zimbabwean study showing a 2-fold increased risk of ARIs in children exposed to biomass fuels[27] and in Cameroonian children, the majority of which were infants, the risk of ARI following indoor air pollution exposure was increased 3.6-fold.[28] The importance of early-life environmental exposures on lung health is highlighted in South African birth cohort study which found that both antenatal indoor air pollution and environmental tobacco smoke increased the risk of LRTI[29] and using genotyping showed that infants carrying asthma-related risk alleles were more susceptible to particulate matter, PM10-associated reduced lung function, highlighting the importance of gene-environment interactions.[30] Further, with childhood TB contributing up to 20% of the caseload in high incidence countries,[31] the impact of environmental exposures on TB incidence must be considered. A systematic review found that while there were too few studies on air pollution and TB to assess causality, the pooled odds ratio of children exposed to environmental tobacco smoke developing TB disease was almost 3-fold, (OR 2.8(95%CI 0.9–4.8).[32]
In terms of chronic respiratory conditions, a high burden of air pollution exposure, chronic respiratory symptoms, and lung function abnormalities were reported[33] and both indoor and outdoor air pollution have been associated with asthma exacerbations in children.[34-36]
Addressing risk factors of LRTI are vital to reduce mortality.[1] The reliance on polluting fuels for household energy in many African communities, contributes to under-5 mortality, requiring urgent public health interventions.[37,38] The current COVID-19 pandemic further highlights the vulnerabilities of many African children and the dire consequences of colliding pandemics.[39] The adoption of “lockdown policies” in many countries, as an attempt to decrease the spread of SARSCoV-2 infection, has potentially inversely increased the risk of other environmental exposures, especially for children confined to suboptimal household living conditions with exposure to indoor air pollution from various sources.[40]
Interestingly, studies from China[41] and Italy[42,43] have shown significant associations between increased outdoor air pollution levels, both short and long term, and the spread of SARS-CoV-2, as well as poorer outcomes and increased mortality. It is postulated that chronic inflammation and dysregulated immune function from chronic air pollution exposure increased the lethality of SARS-COV-2, even in young, healthy people.[43] An Indian study assessing indoor air pollution and tobacco smoke exposure as risk factors for children under-5 acquiring COVID-19 also found an association, again highlighting the importance of reducing exposure to household pollutants.[44] The COVID-19 pandemic brings to the fore the contribution of social inequality to health outcomes[45] and the impact of environmental exposures on both acute and chronic childhood respiratory conditions.[46]
PEDIATRIC LUNG FUNCTION
Lung function is a key in the diagnosis and management of respiratory disease and in epidemiological respiratory health research. Recent advances in lung function equipment and standards have made lung function increasingly feasible in even small children and infants,[47-49] allowing lung health to be assessed and tracked throughout life. Access to lung function testing, particularly in young children, is extremely limited in many parts of Africa, despite the disproportionate burden of respiratory disease and urgency to strengthen diagnosis and management.[50] However, over the past decade, much has happened to strengthen the use of these tools toward improving respiratory health in African children.
Advances in testing capacity and development of normative standards have been made in the past few years. Access to training in spirometry, training materials, quality assurance, and overreading and train-the-trainer programs have been facilitated through the PATS spirometry program (www.panafricanthoracic.org). Over the past 3 years, 450 health care workers have been trained in spirometry testing and interpretation across 18 African countries in all regions, significantly increasing capacity and access to this tool. However, appropriate reference standards are essential for correct interpretation. In 2012, the Global Lung Function Initiative (GLI) (www.lungfunction.org) published the first all age multiethnic reference standard, in an attempt to standardize robust lung function assessment around the world.[51] Recently, this reference has been validated for central African (Madagascar, Angola, and DRC),[52] North (Algeria),[53] and Southern Africa (South Africa)[54] pediatric populations, each showing a different GLI ethnic fit confirming that standards need to be population specific.[55] These papers have provided key data in providing evidence for reference standards for African children.
Infant and preschool lung function, feasible in unsedated children in community settings, has been established in a number of African cohorts and clinics and used to assess impact of antenatal and early-life lung function in African children,[56-58] and contributed to international efforts to improve diagnosis of respiratory disease through improved clinical utility of preschool oscillometry.[49] This has enabled description of lung function in healthy African infants and development of normative ranges for tidal breathing, inert gas multiple breath washout (MBW), exhaled nitric oxide, and oscillometry.[56] Oscillometry is a non-invasive measure of respiratory system resistance and compliance, collected during quite tidal breathing that can be used across the life course. It has been shown to be sensitive to detect impact of antenatal impacts on lung function in African infants,[59] and novel intrabreath oscillometry measures have been found to be more sensitive than standard measures in detecting risk for respiratory illness in infants.[60] Important early determinants of low lung function and increased respiratory disease risk have been identified, including air pollution,[57,61] HIV exposure,[62] and impact of early-life infections.[6] These findings highlight key target areas for strengthening respiratory health. To support development and collaboration across the continent, the African infant and preschool lung function working group has been established (https://www.panafricanthoracic.org/working-groups/african-paediatric-lung-function), including 18 clinicians and clinical researchers working in pediatrics respiratory health representing all African regions. The COVID-19 pandemic has had important planning and cost implications on lung function testing, due to need for increased infection control[63] and increased aerosolizing risk associated with lung function techniques.
It is unclear what impact COVID-19 may have on children with chronic lung disease nor the impact this may have longer term on their lung function trajectory.[64] Monitoring lung function with the same test across the life course will be important to provide this data. Although most children have very minor respiratory disease, older children and adolescents who have had severe COVID-19 pneumonia are likely to need careful follow-up with lung function testing to assist in management planning. Given the growing evidence for COVID-19 to effect pulmonary vasculature as well as lung parenchyma, lung function testing after COVID-19 pneumonia should include measurement of the alveolar-pulmonary vasculature interface, such as measurement of gas transfer (TLCO/DLCO) and measurement of effort tolerance with exercise testing, rather than spirometry or oscillometry alone.
Finally, as we continue to experience limitations in travel and group meetings, ongoing training and support, and even lung function measurement, may have to shift to electronic platforms going forward. Development of e-Learning training will be an important and innovative ways to measure lung function remotely should be explored.
CHILDREN’S RIGHTS AND COVID-19
While children are fortunately spared from severe COVID-19, there are growing concerns that the COVID-19 pandemic will have a devastating and potentially lifelong impact on children’s health and development as governments’ efforts to curb the spread of the virus have disrupted routine services, triggered an economic recession, and intensified existing inequalities.
The health and development of Africa’s children were already under threat before the advent of COVID-19. For example, in 2018, nearly 60% of South Africa’s children lived in poverty, 30% were without access to water on site, and 18% lived in overcrowded households.[5] It is therefore not surprising that preventable conditions such as malnutrition, LRTIs, and acute diarrhea continue to drive under-5 mortality. These pre-existing patterns of adversity have left children vulnerable to shocks such as conflict, climate change, and COVID-19. For example, implementing preventative measures such as handwashing and physical distancing are challenging – if not impossible – in the dense informal settlements of African cities. At the same time, hard lockdown measures have precipitated a sharp increase in unemployment, pushing families deeper into poverty, and increasing the burden of malnutrition, violence, and mental health conditions – at a time when children have limited access to education, health care, and social services. For example, 47% of South African households ran out of money to buy food in early lockdown, with 15% of respondents reporting that a child had gone hungry in the past 7 days.[7]
Recommended steps for safe pediatric lung function testing during COVID-19 pandemic
Avoids unnecessary lung function testing; reserve lung function testing for circumstances where it will influence management decisions.
SARS-CoV-2 infection symptom and contact screening; avoid testing of symptomatic patients.
Increase infection control interventions and practice physical spacing.
Universal personal protective equipment for health professionals and face masking for patients.
Appropriate medical filters and cleaning protocols of lung function equipment.
Preference for tidal breathing measures e.g. oscillometry for bronchodilator response testing, asthma and assessment of chronic obstructive lung disease or MBW for children with cystic fibrosis.
Key child rights priorities for action
Promote child-friendly COVID-19 responses - that minimize disruptions to essential services such as antenatal care, immunization, contraception, nutrition and child protection services
Protect jobs and expand access to social grants, food relief and school feeding programs to protect children from extreme poverty and hunger
Improve children’s access to adequate housing, water and sanitation
Ensure basic education is uninterrupted and access to remote learning supported where needed
Regulate harmful business practices to protect children from exposure to hazardous chemicals, environmental pollution and climate change
Increase taxes on unhealthy products such as alcohol, tobacco and sugar-sweetened beverages, and use these funds to build a more equitable, child-centered and resilient health care system that is better equipped to cope with future shocks and pandemics
The inevitable diversion of scarce resources to adult COVID-19 care has come at the expense of child health services. This includes disruption of routine maternal and child health services; stock-outs of essential medicines; an increase in late presentations and severe complications like disseminated TB due to delays in care-seeking behavior. Immunization coverage in South Africa dropped to 61% in April 2020 (down from 82% the previous year), while a national survey found that 23% of respondents were unable to access chronic medication or contraception in May/June, with 11% of new and HIV+ mothers running out of ARVs.[65,66] Given these multiple threats to children’s health and development, it is vital that children are prioritized in governments’ COVID-19 pandemic response and recovery plans.
Children’s right to health is defined broadly in international law and extends beyond the right to health-care services to include the upstream determinants of child health such as adequate water, sanitation, and housing; an adequate standard of living, and an environment that is not harmful to health.[67] Yet, COVID-19 and future austerity measures threaten to undermine these rights. Strong leadership for child health is therefore essential. As health professionals, we need to draw on our knowledge, skills, and expertise to champion children’s rights to survival, health, and development at facility, district and national level. This includes documenting the direct, indirect, and anticipated effects of COVID-19 on children, identifying threats to child health, and strengthening support systems for children and families – both within and outside the health-care system.
CONCLUSION
Despite prevailing challenges in Africa, there have been significant improvements in overall child health, pneumonia-related mortality, and our understanding of the causes and epidemiology of pediatric respiratory diseases prevalent in Africa. With improving access to basic health-care interventions, improving socioeconomic conditions, and access to new therapies, a shift to focus more attention on early-life prevention and care of non-communicable chronic lung conditions in children and adults will become increasingly important. The COVID-19 pandemic has exposed many deficiencies in health-care infrastructure in African countries and children are regrettably suffering the consequences of these deficiencies. It is important that children’s health and rights remain at the forefront of government policy and planning in navigating the challenges imposed by the COVID-19 pandemic.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: A systematic analysis. Lancet Glob Health. 2019;7:e47-57.
- [CrossRef] [Google Scholar]
- Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871-8.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges of COVID-19 in children in low-and middle-income countries. Paediatr Respir Rev. 2020;35:70-4.
- [CrossRef] [PubMed] [Google Scholar]
- Protecting children in low-income and middle-income countries from COVID-19. BMJ Glob Health. 2020;5:e002844.
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017. A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736-88.
- [CrossRef] [Google Scholar]
- Lung function in African infants in the Drakenstein child health study. Impact of lower respiratory tract illness. Am J Respir Crit Care Med. 2017;195:212-20.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet. 2019;394:757-79.
- [CrossRef] [Google Scholar]
- Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: A nested case-control study of the Drakenstein child health study. Lancet Respir Med. 2016;4:463-72.
- [CrossRef] [Google Scholar]
- Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: A systematic review. Lancet Respir Med. 2015;3:235-43.
- [CrossRef] [Google Scholar]
- Advances in the diagnosis of pulmonary tuberculosis in children. Paediatr Respir Rev. ;2020 In press Available at:
- [CrossRef] [Google Scholar]
- Ten key points about COVID-19 in children: The shadows on the wall. Pediatr Pulmonol 2020
- [CrossRef] [PubMed] [Google Scholar]
- Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health. ;2020
- [CrossRef] [Google Scholar]
- Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733-43.
- [CrossRef] [Google Scholar]
- Environmental factors associated with allergy in urban and rural children from the South AFrican Food Allergy (SAFFA) cohort. J Allergy Clin Immunol. 2020;145:415-26.
- [CrossRef] [PubMed] [Google Scholar]
- GINA 2019: A fundamental change in asthma management: Treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir Soc. 2019;53:1901046.
- [CrossRef] [PubMed] [Google Scholar]
- Acute asthma management during SARS-CoV2-pandemic 2020. World Allergy Organ J. 2020;13:100125.
- [CrossRef] [PubMed] [Google Scholar]
- Randomised controlled trial of the efficacy of a metered dose inhaler with bottle spacer for bronchodilator treatment in acute lower airway obstruction. Arch Dis Child. 2007;92:142-6.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchiectasis and other chronic lung diseases in adolescents living with HIV. Curr Opin Infect Dis. 2017;30:21-30.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal changes in spirometry in South African adolescents perinatally infected with human immunodeficiency virus who are receiving antiretroviral therapy. Clin Infect Dis. 2020;70:483-90.
- [CrossRef] [PubMed] [Google Scholar]
- The lancet commission on pollution and health. Lancet. 2018;391:462-512.
- [CrossRef] [Google Scholar]
- Air Pollution and Child Health: Prescribing Clean Air In: Summary. Geneva: World Health Organization; 2018.
- [Google Scholar]
- An estimate of asthma prevalence in Africa: A systematic analysis. Croat Med J. 2013;54:519-31.
- [CrossRef] [PubMed] [Google Scholar]
- The international study of asthma and allergies in childhood (ISAAC): Phase three rationale and methods. Int J Tuberc Lung Dis. 2005;9:10-6.
- [Google Scholar]
- Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: A systematic review and meta-analysis. Bull World Health Organ. 2008;86:390-8.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory disease associated with solid biomass fuel exposure in rural women and children: Systematic review and meta-analysis. Thorax. 2011;66:232-9.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823-60.
- [CrossRef] [Google Scholar]
- Indoor air pollution from biomass combustion and acute respiratory illness in preschool age children in Zimbabwe. Int J Epidemiol. 2003;32:847-53.
- [CrossRef] [PubMed] [Google Scholar]
- Acute respiratory infection related to air pollution in Bamenda, North West Region of Cameroon. Pan Afr Med J. 2019;32:99.
- [Google Scholar]
- Early-life exposure to indoor air pollution or tobacco smoke and lower respiratory tract illness and wheezing in African infants: A longitudinal birth cohort study. Lancet Planet Health. 2017;1:e328-36.
- [CrossRef] [Google Scholar]
- Genetic susceptibility to asthma increases the vulnerability to indoor air pollution. Eur Respir J. 2020;55:1901831.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014;49:430-4.
- [CrossRef] [PubMed] [Google Scholar]
- Childhood tuberculosis and exposure to indoor air pollution: A systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:596-602.
- [CrossRef] [PubMed] [Google Scholar]
- Lung health and exposure to air pollution in Malawian children (CAPS): A cross-sectional study. Thorax. 2019;74:1070-7.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma-related outcomes associated with indoor air pollutants among schoolchildren from four informal settlements in two municipalities in the Western Cape Province of South Africa. Indoor Air. 2019;29:89-100.
- [CrossRef] [PubMed] [Google Scholar]
- The association between ambient NO2 and PM2.5 with the respiratory health of school children residing in informal settlements: A prospective cohort study. Environ Res. 2020;186:109606.
- [CrossRef] [PubMed] [Google Scholar]
- Effect modifiers of lung function and daily air pollutant variability in a panel of schoolchildren. Thorax. 2019;74:1055-62.
- [CrossRef] [PubMed] [Google Scholar]
- The associations between types of ambient PM2.5 and under-five and maternal mortality in Africa. Int J Environ Res Public Health. 2017;14:359.
- [CrossRef] [PubMed] [Google Scholar]
- Cooking fuel and risk of under-five mortality in 23 Sub-Saharan African countries: A population-based study. Int J Environ Health Res. 2017;27:191-204.
- [CrossRef] [PubMed] [Google Scholar]
- Global lung health: The colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J. 2010;35:27-33.
- [CrossRef] [PubMed] [Google Scholar]
- Health Threats Associated with Children Lockdown in Spain during COVID-19; 2020. Available from: https://www.ssrn.com/abstract=3567670 [Last accessed on 2020 Aug 22]
- [CrossRef] [Google Scholar]
- Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci Total Environ. 2020;727:138704.
- [CrossRef] [PubMed] [Google Scholar]
- Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ Pollut. 2020;264:114732.
- [CrossRef] [PubMed] [Google Scholar]
- Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ Pollut. 2020;261:114465.
- [CrossRef] [PubMed] [Google Scholar]
- Indoor air pollution (IAP) and preexisting morbidities among under-5 children in India: Are risk factors of coronavirus disease (COVID-19)? Environ Pollut. 2020;266:115250.
- [CrossRef] [PubMed] [Google Scholar]
- Why inequality could spread COVID-19. Lancet Public Health. 2020;5:e240.
- [CrossRef] [Google Scholar]
- Indoor air quality and severity of COVID-19: Where communicable and non-communicable preventive measures meet. Asia Pac J Med Toxicol. 2020;9:1-2.
- [Google Scholar]
- An official American thoracic society/European respiratory society statement: Pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304-45.
- [CrossRef] [PubMed] [Google Scholar]
- Preschool multiple-breath washout testing. An official American thoracic society technical statement. Am J Respir Crit Care Med. 2018;197:e1-19.
- [CrossRef] [PubMed] [Google Scholar]
- The international collaboration to improve respiratory health in children (INCIRCLE) ERS clinical research collaboration. Eur Respir J. 2018;52:1801867.
- [CrossRef] [PubMed] [Google Scholar]
- Dealing with access to spirometry in Africa: A commentary on challenges and solutions. Int J Environ Res Public Health. 2019;16:62.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-ethnic reference values for spirometry for the 3-95yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40:1324-43.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the global lung initiative 2012 reference values for spirometry in African children. Am J Respir Crit Care Med. 2016;195:229-36.
- [CrossRef] [PubMed] [Google Scholar]
- The recent multi-ethnic global lung initiative 2012 (GLI2012) reference values don't reflect contemporary adult's North African spirometry. Respir Med. 2013;107:2000-8.
- [CrossRef] [PubMed] [Google Scholar]
- Choosing the better GLI2012 equation in South African population groups. Am J Respir Crit Care Med 2020
- [CrossRef] [PubMed] [Google Scholar]
- An urgent need for African spirometry reference equations: The paediatric and adult African spirometry study. Int J Tuberc Lung Dis. 2019;23:952-8.
- [CrossRef] [PubMed] [Google Scholar]
- Lung function and exhaled nitric oxide in healthy unsedated African infants. Respirology. 2015;20:1108-14.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal household air pollution is associated with impaired infant lung function with sex-specific effects. Evidence from GRAPHS, a cluster randomized cookstove intervention trial. Am J Respir Crit Care Med. 2019;199:738-46.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of prone and supine positions on the regional distribution of ventilation in infants and children using electrical impedance tomography. S Afr J Physiother. 2015;71:237.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory impedance in healthy unsedated South African infants: Effects of maternal smoking. Respirology. 2015;20:467-73.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-breath measures of respiratory mechanics in healthy African infants detect risk of respiratory illness in early life. Eur Respir J. 2019;53:1800998.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic susceptibility to asthma increases the vulnerability to indoor air pollution. Eur Respir J. 2020;55:1901831.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of HIV and antiretroviral drug exposure on lung growth and function over 2 years in an African birth cohort. AIDS. 2020;34:549-58.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendation from ERS Group 9.1 (Respiratory Function Technologists, Scientists) Lung Function Testing during COVID-19 Pandemic and Beyond. 2020. Available from: https://www.artp.org.uk/write/MediaUploads/Standards/COVID19/ERS_9.1_Statement_on_lung_function_during_COVID-19_Version_1.0.pdf [Last accessed on 2020 Sep 14]
- [Google Scholar]
- Lung function testing in the COVID-19 endemic. Lancet Respir Med. 2020;8:666-7.
- [CrossRef] [Google Scholar]
- Dramatic Drop in South Africa's Immunisation Rates. 2020. Available from: https://www.spotlightnsp.co.za/2020/06/24/dramatic-drop-in-sas-immunisation-rates [Last accessed on 2020 Aug 24]
- [Google Scholar]
- Measuring the Public Health Cost of COVID-19 Control Efforts. 2020. Available from: https://www.cramsurvey.org/wpcontent/uploads/2020/07/Burger-examining-the-unintended-health-consequences.pdf [Last accessed on 2020 Aug 24]
- [Google Scholar]
- African Charter on the Rights and Welfare of the Child. 1990. OAU Doc. CAB/LEG/24.9/49. Available from: https://www.un.org/en/africa/osaa/pdf/au/afr_charter_rights_welfare_child_africa_1990.pdf [Last accessed on 2020 Sep 14]
- [Google Scholar]






