Translate this page into:
Asthma control and use of short-acting beta-2-agonists for symptom relief in Africa – A narrative review
*Corresponding author: Sandra Kwarteng Owusu, Department of Child Health and Paediatrics, School of Medical Sciences Kwame Nkrumah University of Science and Technology Kumasi Ghana. abenaboamah18@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chakaya JM, Kwarteng Owusu S, Smith C. Asthma control and use of short-acting beta-2-agonists for symptom relief in Africa – A narrative review. J Pan Afr Thorac Soc. 2024;5:57-61. doi: 10.25259/JPATS_10_2024
Abstract
More than 100 million people in Africa live with Asthma. With appropriate treatment, predominantly inhaled corticosteroids (ICSs), asthma is controllable in most people. However, in Africa, multiple factors constrain the accessibility, availability, and quality of asthma services, which lead to underdiagnosis and sub-optimal treatment of the disease. These constraints include weak healthcare systems and inadequate knowledge of healthcare providers on asthma diagnosis and treatment. There is also a poor awareness of asthma as a long-term disease in the general population. There are several myths and misconceptions about asthma as a disease. There is also poor access to ICSs, the most effective controller medicines for asthma. This is mostly due to the high cost. Furthermore, the non-use or delays in initiating treatment with inhaled steroids commonly leads to overuse and overreliance on rapid relievers of asthma symptoms, and in particular, short-acting beta-2-agonists (SABAs), both in oral and inhaled forms. This practice is very common and contributes to the sub-optimal treatment and resultant poor control of asthma, which is highly prevalent in Africa. In this paper, we highlight the burden of asthma in Africa, the effects of overuse/overreliance of SABA use on asthma control and provide suggestions to reduce the dependency on SABAs to improve asthma treatment and enhance asthma control on the African continent.
Keywords
Asthma
Short-acting beta-agonist
Asthma control
Africa
INTRODUCTION
Asthma is a serious global health problem estimated to affect more than 226 million people worldwide and over 119 million people on the African continent.[1] Inhaled corticosteroids (ICS), taken regularly or as needed when combined with short-acting beta-2-agonists (SABAs) (anti-inflammatory reliever therapy or AIR), represent the cornerstone of asthma management across all severities, while SABAs only provide rapid symptom relief.[2] SABAs have no inherent anti-inflammatory activity, and their overuse (≥3 canisters/year) is associated with an increased incidence of exacerbations, mortality, and healthcare costs.[3-6]
ASTHMA CARE IN AFRICA
Asthma care in Africa is occurring in the background of high levels of poverty and weak health care systems. Inequities in access to healthcare care are common across Africa and are particularly severe among certain populations, such as those living in urban slums and rural communities. At present, nearly half a billion people in Africa are estimated to be living in extreme poverty.
The African region is unfortunately experiencing uneven progress toward the achievement of the United Nations 2030 target for sustainable development goal (SDG) 1, which is to end poverty in all forms.[7] Poverty has been associated with a higher burden of asthma, including asthma deaths.[8,9] It is, therefore, not entirely surprising that asthma control has not been achieved for a large proportion of people living with asthma in Africa. Poverty should, however, not be an excuse for the lack of optimal asthma control in Africa.[10]
CURRENT STATE OF MANAGEMENT OF ASTHMA AND USE OF SABAs TO MANAGE ASTHMA
It is safe to state that, in general, asthma care and management in Africa is sub-optimal for adults, adolescents, and children. Of the 26,654 individuals with doctor-diagnosed asthma who participated in the global asthma network phase 1 study in which three African countries (South Africa, Cameroon, and Nigeria) contributed participants, between 29.3% and 85.3% used SABAs alone, only 12.6–51.9% used inhaled steroids (ICS), and 44.8% individuals with severe asthma were not using ICS[11] [Table 1].
| 1 | Out of a total of 26,654 participants from South Africa, Cameroon and Nigeria, between 29.3% to 85.3% used SABA alone |
| 2 | Nearly fifty percent of participants did not use inhaled corticosteroids (ICS). |
| 3 | Forty-four percent of participants with severe asthma did not use ICS |
In addition, in a recent systematic review by Mphahlele et al. that included 883 children and adolescents from Nigeria, South Africa, and Uganda, the prevalence of uncontrolled asthma ranged from 30.9% to 44.5%. Regular use and adherence to ICS were poor, with less than 10% of the participants from Uganda (screening done in the community) reporting that they had used ICSs routinely in the past 12 months. Further, among the cohort of children and adolescents with uncontrolled asthma, the majority reported that they preferred to use only SABA.[12]
Overuse of SABAs is a common global asthma care challenge.[13] The SABA use in Asthma (SABINA) studies have provided concerning information on the use of SABAs in Africa. Three African countries (South Africa, Kenya, and Egypt) were involved in the SABINA III study, which revealed a dismal state of affairs with nearly half of all patients (46.5%) from the African cohort prescribed ≥3 SABA canisters in the past 12 months, which is considered over-prescription.[14] In both the Kenyan and South African cohorts, over 70% (71.9% in Kenya and 75% in South Africa) of patients were prescribed 3 or more SABA canisters in 1 year.[15,16] More than 25% purchased the SABA without a prescription in South Africa, and while the figure stood at 38.8% in Kenya, in Africa as a whole, the figure was 32.6%[14-16] [Table 2].
| 1 | Nearly half of all participants (46.5%) in the African cohort were prescribed>3 canisters of SABA in a 12-month period (over prescription) |
| 2 | More than a quarter of all participants purchase SABA over a counter without prescription in all three African countries |
Based on robust clinical trial evidence, the Global Initiative for Asthma (GINA) no longer recommends as-needed SABA without concomitant ICS for adults and children above 6 years of age.[2] The use of SABA alone and or excessive use of SABA in the treatment of asthma has been associated with poor asthma control, higher rates of asthma exacerbations, and asthma deaths.[6,17] Asthma exacerbations and deaths occur even when the asthma is considered to be mild by both the healthcare provider and the person with asthma.[3] Thus, there is a major imperative to reduce the rate of asthma exacerbations and, therefore, prevent asthma deaths in all people with asthma, including those with infrequent symptoms. This was the focus of the key studies that informed the decision by GINA to recommend the change from using SABA alone for relief of asthma symptoms in persons with infrequent symptoms to using an ICS with formoterol as needed or ensuring an ICS is also taken whenever SABA is used.[3,13,18,19] The documented overuse of SABA on the African continent is, therefore, a major cause of concern.
It is important to ask the question, “Why is there overuse of SABA in Africa?” There are multiple reasons for the overuse of SABA in Africa and elsewhere in the world. SABAs have been used for a long time and are on the minds of many healthcare providers and people with asthma with their work. Asthma symptoms rapidly resolve when these medicines are used. Because they do not prevent the next attack, used alone, these medicines are inappropriate for the long-term control of asthma. Compared with ICS, SABAs are considered affordable by people with asthma, healthcare providers, and healthcare systems. This makes these medicines more attractive to healthcare financiers, especially governments, in the low-resource settings of Africa. A myopic view of the cost of treatment of asthma based on the unit cost of medicines may be contributing to the documented poor access to ICS in Africa.[20] The widespread myths and misconceptions about asthma and the fear of “steroids” are also likely to be contributing to the overuse of SABAs. The fact that these medicines are freely available without a prescription in the African medicine market is also likely to be contributing to their overuse [Table 3].
|
WHAT CAN BE DONE TO REDUCE THE OVERUSE OF SABA IN AFRICA?
Reducing SABA overuse will not be an easy task; however, efforts must be made to achieve this goal if the asthma control landscape in Africa is to change. We suggest that the following actions be undertaken in the effort to reduce SABA overuse in Africa: [Figure 1].
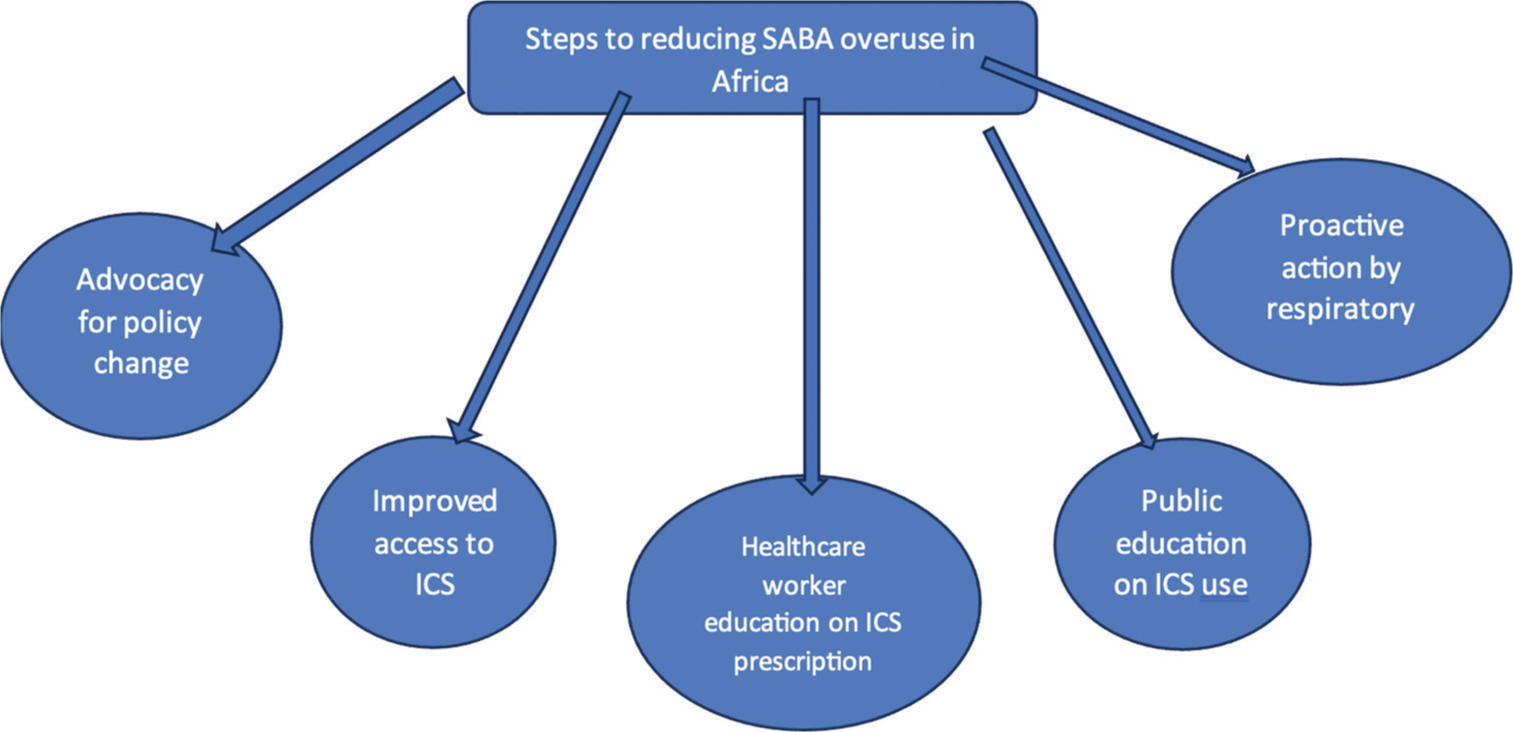
- Steps to reducing short acting beta-2- agonist (SABA) over use in Africa. ICS: Inhaled corticosteroids.
Advocacy for policy change
In many African countries, there are no country-specific asthma guidelines, which means healthcare providers make asthma care choices in a largely unknown way. We postulate that the more learned healthcare providers may be guided by global recommendations that are made by organizations such as GINA, the World Health Organization (WHO), and the International Union Against TB and Lung Diseases (the Union) or professional societies with a global reach such as the European Respiratory Society and the American Thoracic Society. While the GINA recommendations are updated every year, the asthma care recommendations by the WHO have not been updated for many years and still recommend the use of SABA alone for the relief of symptoms in those with mild disease.[21] We believe that every person with asthma in the African continent should have access to the best treatment that is currently recommended by GINA. For this to be achieved in national asthma care, recommendations that fit with global norms will need to be developed and adopted as national policy. To get governments to begin to pay serious attention to asthma, data on asthma morbidity and mortality will need to be collected and used to advocate for better asthma care. To counter arguments over the cost of ICS with or without other molecules, the data obtained on asthma morbidity and mortality could be used to model pathways for asthma care and to demonstrate that asthma treatment with ICS is cost-effective and good value for money. Talking sensibly to governments about money for asthma care is likely to make public health care policymakers and public health program developers see the value in investing in asthma care programs. It is gratifying that a trial of ICS with formoterol (AIR-SA) is currently ongoing in South Africa. This study is expected to provide critical data on the use of ICS/formoterol in the African setting.
It is important that policymakers understand that the widespread availability of SABAs in pharmacies without a prescription is likely to be hurting people with asthma rather than helping them. Therefore, we strongly advocate for these medicines to be made prescription only products. Limitation of access to SABAs will need intervention by local medicine controlling bodies, possibly including a “black box” warning in patients with asthma.
Healthcare provider education and general public awareness
A major reason for the overuse of SABAs in the African continent is the low level of awareness of current asthma care recommendations by healthcare providers, in addition to low levels of awareness about asthma in the general public.
To address this, educational programs for both healthcare providers and the general public must be undertaken so that widespread adoption of current evidence-based recommendations for asthma treatment, including the use of AIR therapy, can be taken up and prioritized by all stakeholders. Educational programs must include all cadres of healthcare workers (doctors, nurses, and pharmacists), people living with asthma, and the wider community. In a low-income setting, patients may frequently consult peripheral clinics staffed by nurses or ask the pharmacist for advice. This presents an opportunity for targeting these groups, as well as doctors, with ongoing educational programs. The clinicians in each country would be best placed to drive these initiatives.
Asthma drug market shaping
Pan-African asthma stakeholders (people with asthma, health-care providers with an interest in this disease, public health programs that coordinate care for lung diseases, and others) need to come together to address access to recommended asthma medicines on the African continent. Engagement with the pharma industry leading to negotiated prices of essential asthma medicines may yield dividends. This may include volume-based pricing of these medicines.
Proactive action by respiratory societies
To support the achievement of the United Nations 2030 SDG 3, which aims at “ensuring healthy lives and promoting well-being,” we call to action all respiratory societies in each country and the Pan African Thoracic Society to help drive the process of change with educational programs targeting health-care providers and the general public and to lobby governments to prioritize asthma and to provide optimized asthma care for all. Respiratory societies should also lobby local medicine control councils to limit the availability of SABAs.
CONCLUSION
Asthma is a major public health concern with a rapidly increasing burden in Africa. Its treatment is currently suboptimal with overuse of SABA, which leads to poor asthma control with an increased risk of asthma exacerbations and deaths. This situation needs redress urgently through advocacy efforts, educational programs, and market shaping. We urge respiratory societies across Africa to take the lead in pushing for change so that asthma deaths are brought to zero on the continent.
Authors’ contributions
All authors contributed equally to the development of this paper.
Declaration of interest
This paper is based on presentations made by the authors at the Pan African Thoracic Society webinar on asthma held on November 30, 2023. This webinar was supported by Astra Zeneca; however, the authors confirm that the views expressed in this paper are their own and do not represent those of Astra Zeneca. JC and CS were the principal investigators for the SABINA III study in Kenya and South Africa, respectively.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- An estimate of asthma prevalence in Africa: A systematic analysis. Croat Med J. 2013;54:519-31.
- [CrossRef] [Google Scholar]
- The paradoxes of asthma management: Time for a new approach? Eur Respir J. 2017;50:1701103.
- [CrossRef] [Google Scholar]
- SABINA: Global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur Respir J. 2020;55:1901858.
- [CrossRef] [Google Scholar]
- Asthma-related health outcomes associated with short-acting β2-agonist inhaler use: An observational UK study as part of the SABINA global program. Adv Ther. 2020;37:4190-208.
- [CrossRef] [Google Scholar]
- Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: A nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55:1901872.
- [CrossRef] [Google Scholar]
- 2018 Overcoming poverty and inequality in South Africa: An assessment of drivers, constraints and opportunities In: World Bank.
- [Google Scholar]
- Pediatric asthma health disparities: Race, hardship, housing, and asthma in a national survey. Acad Pediatr. 2017;172:127-34.
- [CrossRef] [Google Scholar]
- Socioeconomic factors associated with asthma prevalence and severity among children living in low-income South African communities. S Afr Med J. 2016;106:57.
- [CrossRef] [Google Scholar]
- Asthma control and its predictors in Ethiopia: Systematic review and meta-analysis. PLos One. 2022;17:e0262566.
- [CrossRef] [Google Scholar]
- Asthma management and control in children, adolescents, and adults in 25 countries: A Global Asthma Network Phase I cross-sectional study. Lancet Glob Health. 2023;11:e218-28.
- [CrossRef] [Google Scholar]
- Barriers and determinants of asthma control in children and adolescents in Africa: A systematic review. BMJ Open. 2021;11:e053100.
- [CrossRef] [Google Scholar]
- Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: The multi-country, cross-sectional SABINA III study. Eur Respir J. 2022;59:2101402.
- [CrossRef] [Google Scholar]
- Over-prescription of short-acting β2-agonists is associated with poor asthma outcomes: Results from the African cohort of the SABINA III study. Curr Med Res Opin. 2022;38:1983-95.
- [CrossRef] [Google Scholar]
- Over-prescription of short-acting β2-agonists for asthma in South Africa: Results from the SABINA III study. Afr J Thorac Crit Care Med. 2022;28:172-80.
- [CrossRef] [Google Scholar]
- Over-prescription of short-acting β2-agonists remains a serious health concern in Kenya: results from the SABINA III study. BMC Prim Care. 2023;24:141.
- [CrossRef] [Google Scholar]
- The national review of asthma deaths: What did we learn and what needs to change? Breathe. 2015;11:14-24.
- [CrossRef] [Google Scholar]
- Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): A 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919-28.
- [CrossRef] [Google Scholar]
- Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380:2020-30.
- [CrossRef] [Google Scholar]
- The reality of managing asthma in subSaharan Africa-Priorities and strategies for improving care. J Pan Afr Thorac Soc. 2022;3:105-20.
- [CrossRef] [Google Scholar]
- WHO package of essential noncommunicable (PEN) disease interventions for primary health care. 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/334186/9789240009226.eng.pdf? [Last accessed on 2024 Aug 25]
- [Google Scholar]






