Translate this page into:
Trends of Xpert MTB/RIF in the diagnosis of Mycobacterium tuberculosis and rifampicin resistance in Southwest Nigeria: A 4-year retrospective study
*Corresponding author: Michael Abel Alao, Department of Pediatrics, College of Medicine University of Ibadan and University College Hospital, Ibadan, Oyo, Nigeria. mikevikefountains@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Alao MA, Ibrahim OR, Ogunbosi BO. Trends of Xpert MTB/RIF in the diagnosis of Mycobacterium tuberculosis and rifampicin resistance in Southwest Nigeria: A 4-year retrospective study. J Pan Afr Thorac Soc 2023;4(1): 31-41.
Abstract
Objectives:
In recent years, there has been an increased uptake of Xpert Mycobacterium tuberculosis/rifampicin (MTB/RIF) for the diagnosis of tuberculosis (TB), with added benefits for detecting rifampicin-resistant TB (RR-TB). We set out to determine the trends in notification of MTB and RR-TB over 4 years in a tertiary hospital in Southwest Nigeria.
Materials and Methods:
This is a retrospective analysis of single early morning sputum/gastric washing sample for presumed TB in a tertiary health center between January 2016 and December 2019. Xpert MTB/RIF was used to detect (MTB) and RR-TB.
Results:
The mean age of the 4625 presumptive TB patients was 43.4 (18.8) years, with the majority aged 31–45 (30.6%). Males totaled 2247 (49.6%). Human immunodeficiency virus (HIV) coinfection was found in 756 (16.7%) of them. Using the Xpert MTB/RIF assay, the overall MTB notification was 12.9% (584/4526) and was associated with HIV status, P < 0.001. MTB notification trends increased from 5.6% in 2016 to 23.8% in 2019, P < 0.001. The overall yield of RR-TB was 7.5%. The proportion of RR-TB from TB cases declined from 28% in 2016 to 4.6% in 2019. Forty-two of the 44 cases of the RR-TB (42/572; 7.3%, [95% CI: 5.31, 9.75]) were new cases, with TB treatment failure and relapse cases accounting for 10.1% (2/11; 10.1%, [95% CI: 0.35, 42.5]) of all RR-TB cases (P < 0.01). RR-TB was associated with being older than 45 years (adjusted odds ratio = 2.046, [95% CI: 1.046, 4.004]). HIV infection status and gender had no effect on RR-TB status.
Conclusion:
This study found an increase in MTB detection with Xpert MTB/RIF utilization. Ages >45 years have 2–4-fold increased risk of developing RR-TB and should be targeted for drug resistance prevention.
Keywords
Tuberculosis
Rifampicin resistance tuberculosis
Multidrug-resistant tuberculosis
Xpert Mycobacterium tuberculosis/Rifampicin
Nigeria
INTRODUCTION
Tuberculosis (TB) is a communicable disease caused by the bacterium, Mycobacterium tuberculosis (MTB).[1] It remains a global public health challenge, posing a growing concern in the developing countries, Nigeria inclusive.[2-5] The increasing burden of drug-resistant TB (DR-TB), which can manifest as isoniazid-resistant TB, rifampicin-resistant TB (RR-TB), multidrug-resistant TB (MDR-TB), pre-extensively DR-TB, and extensively DR-TB, is particularly concerning.[3]
In 2019, the World Health Organization (WHO) global TB report indicated that 1.7 billion people are at risk of TB, with an estimated 10 million new cases every year.[3] Although notification of TB cases appears to be improving from 6.4 million new cases of TB in 2017–7.0 million in 2018, the actual burden of TB in the developing countries remains largely unknown.[3-5] The suggested reasons include under-reporting and challenges with diagnosis, as two in every three cases of DR-TB go undiagnosed and thus are not reported.[3]
In most developing countries, TB is still diagnosed based on the demonstration of the presence of acid fast bacilli (AFB) in clinical samples, such as sputum stained with Ziehl– Neelsen (ZN) staining.[6] The microscopic confirmation of MTB in such smears is observer dependent and useful mainly in advanced disease. Furthermore, high false-negative results are typical with a small quantity of the organism and sensitivity range from 6% to 80%.[7] In contrast, rapid molecular tests using the Xpert MTB/RIF have a sensitivity of 98.6% for MTB, reduced overall turnaround time to as low as 2 h compared with AFB smear which may last 3 days and Lowenstein-Jensen culture, 6 weeks.[7,8] The Xpert MTB/RIF test demonstrated a high diagnostic yield for TB.[9] Among the rapid molecular tests recommended by WHO, the Xpert MTB/RIF, which runs on Cepheid’s Xpert automated polymerase chain reaction (PCR) platform is preferred. The rapid turnover of 2 h, lack of need for extensive training for personnel conducting the test, and higher sensitivity and specificity in detection of MTB and rifampicin resistance TB make the Xpert MTB/RIF® the preferred choice for diagnosis of TB based on the WHO recommendations.[7,10]
Globally, the trend of MTB diagnosis has increased with a surge between 2017 and 2019.[11] A similar pattern of sharply rising disease notifications was observed in Africa, Southeast Asia, and Latin America, albeit to a lesser extent.[12] This improved notification has been attributed to increased utilization of molecular rapid molecular tests and other newer testing modalities.[2,4]
In addition to MTB notification trends, monitoring anti-TB drug resistance is essential for achieving the 2035 END TB goal. This is accomplished through routine diagnostic drug susceptibility testing utilizing rapid molecular tests, culture methods, or sequencing technologies.
According to a recent systematic review and meta-analysis conducted by Onyedum et al.[13] in 2017, the prevalence of DR-TB among newly diagnosed cases in Nigeria was 32%, while the prevalence rate among previously treated cases was 53.0%. The reported prevalence of 32% is significantly higher than the global prevalence of 3.4% reported for new cases in the 2019 WHO global TB report, reaffirming Nigeria as one of the high burden countries for DR-TB.[2,9] Undernutrition, diabetes, smoking, harmful alcohol consumption, poor treatment adherence to anti-TB drugs; previous haphazard or poor implementation of isoniazid prophylaxis and previous ineffective TB treatment, especially in private practice; increasing treatment failure and loss to follow-up, poor Xpert MTB/RIF diagnostic coverage; and a high burden of human immunodeficiency virus (HIV) coinfection in low-resource settings, including Nigeria, are potential drivers for the high prevalence of resistant TB.[13-22] Higher prevalence of social determinants such as poverty, inequality, unsafe housing, discrimination, and social stigma in low-resource settings such as Nigeria also plays a significant role in the spread of DR-TB.[13,14,20]
According to the 2019 WHO global TB report, Nigeria has one of the highest burdens of MDR/RR-TB, accounting for 4.2% of new cases and 15% of previously treated MTB.[2] The reported figure is comparable to those from South Africa, but higher than the regional average and lower than Somalia’s notification rate of 8.7% from new cases. Southeast Asia has roughly half as many MDR/RR-TB cases from new and old cases as Nigeria.[4] While a higher proportion of MDR/RRTB in newly diagnosed cases was observed in Nigeria, disease notification of MDR/RR-TB in previously treated TB cases in the Americas and Nigeria was identical.[2]
In terms of global trends, there appears to be a reassuring reduction in MDR/RR-TB cases between 2015 and 2018. The 2019 WHO global report indicates a 10% decrease in MDR/RR-TB cases with a wide confidence interval (CI). While countries such as Myanmar, Peru, and the Russian Federation experienced an increase, Zambia in Africa and Kyrgyzstan in Asia have observed a decline of approximately 3% per year.
As the world strives to meet the high-level UN TB treatment targets and the WHO End TB Strategy by 2035 benchmarks, the use of rapid molecular tests for early diagnosis and sensitivity testing is critical to achieving these goals, in addition to addressing the disease’s social determinants.
This study, therefore, aimed at tracking the trends of MTB and the yield of rifampicin resistance TB using Xpert MTB/RIF over a 4-year period at a tertiary health facility in Southwest Nigeria. It is hoped that the results would contribute to MTB surveillance and serve as an indicator of the TB program’s performance.
MATERIALS AND METHODS
Study design and location
This was a cross-sectional and retrospective study of single early morning sputum/gastric washing sample from presumptive TB cases from January 2016 to December 2019 at Bowen University Teaching Hospital (BUTH); a faith-based private teaching hospital in Ogbomosho, Oyo State, Southwest Nigeria. The hospital serves as a regional diagnostic center for all presumptive TB cases seen in the hospital or referred from primary health centers, general hospitals, and other tertiary health facilities in the nearby states. Besides this, we used geographic information system (GIS) software (ArcGIS 10.8) to map the subjects’ geographic locations.
Study settings and coverage for TB diagnosis and treatment
Oyo state had approximately 1397 registered healthcare facilities serving 441,031 people as of 2019, with 784 (56.1%) of them being public facilities.[23] The diagnosis and treatment of TB is provided free of charge to individuals of all ages by the Federal Government of Nigeria, Ministry of Health’s national TB and Leprosy Control Program in collaboration with its supporting partners; the Damian foundation in the Netherlands. Over time, TB treatment coverage has steadily increased. The number of directly observed treatment short-course (DOTS) facilities in Oyo State has increased by 82.8%, from 232 in 2016 to 424 in 2019. The participation of private facilities in TB treatment in the state has increased from 22 in 2016 to 213 in 2019, representing an 868.1% increase. During the same period, the public sector DOTS centers increased by 155.7%, from 97 to 248. The number of facilities offering the Xpert MTB/RIF test has increased from eight in 2016 to 10 in 2021. In communities where the gold standard of rapid molecular diagnosis recommended by the WHO is not available, centers designated to provide microscopy services for acid-fast bacilli are available throughout the State’s Local Government Areas. At present, in the state, a 21.4% increase in microscopy services for MTB has been observed (from 56 to 68 centers). The changes observed at the state level parallel the advancements made in understudied local government. In 2016, Ogbomoso had 38 DOTS facilities, 10 of which could perform microscopy tests and two of which were designated as referral centers for the Xpert MTB/RIF test. In 2019, the number of facilities designated to perform microscopy services had increased by 60.5%, reaching 68 from 56. Coverage within the private sector increased from 2 to 24. [Table 1] provides additional details on TB treatment services in the state.
| Types of services | Oyo state | Ogbomoso | ||
|---|---|---|---|---|
| 2016 | 2019 | 2016 | 2019 | |
| Total number of health facility | –* | 1,397 | –* | 219 |
| The number of directly observed therapy centers both public and private | 119 | 461 | 36 | 61 |
| Total number of private health facilities | –* | 613 | –* | 91 |
| Number (%) of DOTS facilities | 22 | 213 (34.7%) | 2 | 24 (26.3%) |
| Total number of public health facilities | –* | 784 | –* | 128 |
| Number (%) of DOTS facilities | 97 | 248 (31.2%) | 36 | 37 (28.9%) |
| Microscopy centers | 56 | 68 | 10 | 10 |
| GeneXpert MTB/RIF | 8 | 2 | 2 | |
| Line probe assay (FLD) sites | 0 | 0 | 0 | |
| Line probe assay (SLD) | 1 | 0 | 0 | |
| Cultures | 1 | 0 | 0 | |
| Drug sensitivity testing | 1 | 0 | 0 | |
Sample size estimation
The minimum sample size required for this study was determined using the Raosoft software (Raosoft Inc., Seattle, Washington, USA, http://www.raosoft.com/samplessize.html). Using the 19.0% MDR/RR-TB national figure of estimated percentage of previously treated cases from the WHO 2019 global TB report (this was chosen to obtain an optimal sample size), a minimum sample size of 4564 was obtained for the study at 80% power, alpha level of probability of 0.05, and margin of error of 1.0%. About 10% of the minimum sample size was added to the estimated sample size to account for missing data.
Study population and eligibility criteria
The study population consist of children and adults with presumptive diagnosis of TB. The details of eligibility criteria are stated below:
Inclusion criteria
The following criteria were included in the study:
All patients with presumed TB, including those with HIV coinfection
Previously treated TB including treatment failure and relapse
Presumed DR-TB
Contact with persons with DR-TB.
Exclusion criteria
The following criteria were excluded from the study:
Indeterminate results
Incomplete results.
Sample collection and laboratory analysis
Each presumptive TB case submitted a single early morning sputum sample (or gastric washing sample collected in the hospital for children unable to produce sputum) into a 25 ml leakproof, wide-mouthed screw cap cup for Xpert MTB/RIF test. For PCR, the sample was diluted 1:2 (v/v) with carbonic acid buffer, vigorously mixed, and incubated at room temperature for 10 min to complete liquefaction. A 2 ml aliquot of the diluted specimen was loaded into the GeneXpert machine using the supplied cartridge port (Xpert MTB/RIF® Sunnyvale, CA, USA). The DNA is released during the mycobacterium’s ultrasonic lysis. The Xpert MTB/RIF utilizes real-time PCR technology to extract, purify, amplify, and quantify DNA using an integrated microfluidic system.[24] In addition, it utilizes a molecular beacon to detect mutations in the rpoB gene associated with RR-TB. At 2 h, the results are read. Quality control is established by first standardizing the instrument with the standard through a check control on the probe. Internal quality control is further enhanced by the sample processing control mechanism. Subjects who tested positive are referred to the Damien Foundation supported TB treatment center in the hospital for appropriate intervention.[25]
Parallel testing for HIV-1 and HIV-2 was performed on all patients with presumptive TB using a first screen with Determine® Kits (Alere Medical, Matsuhidai, Matsudoshi, Chiba-ken Japan) and reactive tests confirmed with the Uni-Gold® HIV (Trinity Biotech, Wicklow, Ireland), with a tiebreak using STAT-PAK® (Chembio Diagnostic System, Medford NY, USA).
Variables
The outcome variables were mycobacterial TB detection and RR-TB identification using Xpert MTB/RIF. For mycobacterial TB Xpert MTB/RIF test results, each outcome variable was dichotomized as either MTB detected or MTB not detected. In the same vein, RR-TB was also dichotomized as either RR_TB detected or not detected. The sociodemographic and clinical variables of age, sex, HIV status, and presumptive TB status at Xpert MTB/RIF screening were the independent variables. Descriptive statistics such as frequency and percentage were used for the present study variables.
Data collection and measured indices
Data collection
One of the authors was involved with activities of the TB treatment program at the facility. The presumptive TB registers designed by the Federal Ministry of Health’s National TB and Leprosy Control Programme and the service laboratory that houses the GeneXpert machine served as the primary data source for this study. Additional information was obtained from BUTH TB treatment registers and the treatment registers from the referral centers. Missing data that were not available at the BUTH were obtained from the State TB and Leprosy Control Programme in Ibadan.
Measured indices
The prevalence of MTB is defined as the number of presumed cases in whom MTB was detected using the Xpert MTB/RIF test (i.e., number of MTB detected in presumed TB cases by Xpert MTB/RIF test/total number of presumed TB cases).
The prevalence of RR-TB among the newly diagnosed is defined as the proportion of new cases with RR-TB relative to the total number of new cases of MTB.
The prevalence of RR-TB among the previously treated MTB cases is defined as the proportion of previously treated MTB cases with RR-TB relative to the total number of previously treated cases of MTB.
Operational definitions
Isoniazid-resistant TB: TB that is resistant to only isoniazid.
RR-TB: TB that is resistant to only rifampin.
MDR-TB: This is defined as TB resistant to at least rifampicin and isoniazid.
Pre-extensively drug resistant: MDR-TB with resistance to a fluoroquinolone or any of the three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin).
Extensively drug-resistant TB: MDR-TB is resistant to rifampicin, as well as any fluoroquinolone, as well as at least one of the drugs bedaquiline and linezolid.
Relapse: A positive smear after a patient has been declared cured.
Treatment failure: The patient is still smear positive 5 months after starting anti-TB treatment.
Default: The patient received at least 1 month of anti-TB treatment with a 2-month break.
Data analysis
Data[26] were entered into a Microsoft Access file and analyzed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA). Descriptive statistics were presented as a frequency table to summarize demographic data (age groups and gender) and HIV status. The proportion of people who tested positive for MTB and RR-TB (RR-TB) was also presented as frequency tables. The association between mycobacterial TB notification using Xpert MTB/RIF and RR-TB and the independent sociodemographic and clinical variables of age, sex (male and female), HIV status (infected, uninfected, and unknown), and presumptive TB status at Xpert MTB/RIF screening (new cases, treatment failure, relapse, and MDR-TB contact) was explored using bivariate analysis. Multivariable logistic regression was used to determine the adjusted odds ratios (AORs) and 95% CIs, for the outcome variables of mycobacterial TB and rifampicin-resistant detection, for sociodemographic and clinical variables that were statistically significant on bivariate analysis. Trends in RR-TB were explored using the Chi-square test for linear trends over the study period and were displayed using line graphs. The level of significance was set at P < 0.05.
Missing data and handling bias
The registers of the TB treatment center were examined and the available data were used to update the dataset. In the final analysis, only missing data not retrievable from the TB registry and not linked to the outcome variable was omitted.
Ethics Approval
The Human Research Ethics Committee of the BUTH granted ethical approval with approval number BUTH-REC – 047. The research was conducted in accordance with the Declaration of Helsinki.
RESULTS
Of the 4625 presumed cases evaluated for TB using the Xpert MTB/RIF test, we excluded 99 (2.1%) because of incomplete data. The flow diagram for participant enrolment is detailed in [Figure 1]. The mean age (standard deviations) of the presumed TB cases was 43.4 (18.8) years, with a range from 3 months to 102 years. Most of the presumptive TB cases were in the age group, 31–45 (30.6%) while children and adolescents (<18 years) were 349 (7.7%). Males were 2247 (49.6%), as shown in [Table 2]. Of the 4625 presumptive TB cases, 756 (16.7%) were living with HIV infection, 2443 (54.0%) were HIV uninfected, and in the remaining 1327 (29.3%), their HIV status was unknown. Based on the distribution, most presumptive TB cases 4436 (98.0%) were new TB cases (no prior TB diagnosis nor treatment), 50 (1.1%) were cases of TB relapse, 16 (0.4%) were TB treatment failure cases, and 24 (0.5%) had a history of contact with DR-TB cases [Table 2].
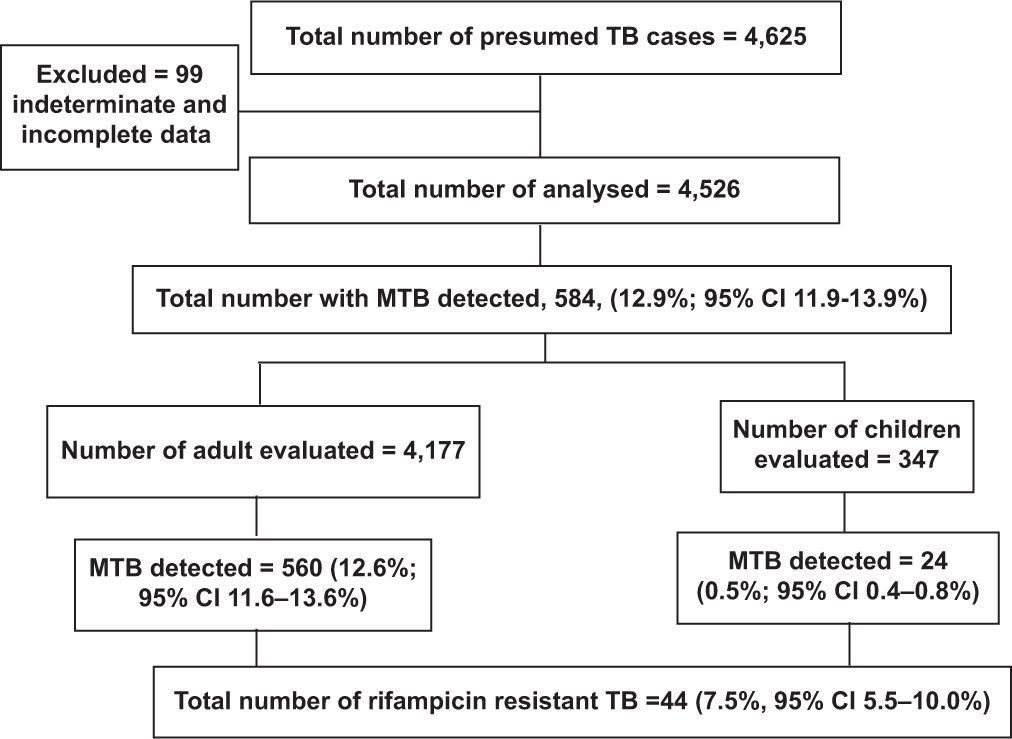
- Flowchart for screening of patients with presumed tuberculosis and rifampicin resistance.
| Variables | Frequency | Percentages |
|---|---|---|
| Age group (years) | ||
| <18 | 349 | 7.7 |
| 18-30 | 877 | 19.4 |
| 31-45 | 1385 | 30.6 |
| 46-60 | 1092 | 24.1 |
| 61-75 | 625 | 13.8 |
| >75 | 198 | 4.4 |
| Sex | ||
| Males | 2247 | 49.6 |
| Females | 2279 | 50.4 |
| HIV status | ||
| Infected | 756 | 16.7 |
| Uninfected | 2443 | 54.0 |
| Unknown | 1327 | 29.3 |
| TB status* | ||
| New cases | 4436 | 98.0 |
| Treatment failure | 16 | 0.4 |
| Relapse | 50 | 1.1 |
| MDR-TB contact | 24 | 0.5 |
TB: Tuberculosis, MDR-TB: Multidrug-resistant tuberculosis.
The overall yield of MTB by Xpert MTB/RIF assay was 12.9% (584/4526). The yield was comparable in males (13.5%; 302/2247) and females (12.4%; 282/2279), χ2 = 1.145, P = 0.288). Based on the age group, the highest yield of MTB was detected in the age 18–30 years (16.2%), while the least was in age <18 years (6.9%; P = 0.002), as shown in [Table 3]. Age <18 years was less likely than adults to have an MTB detected by Xpert MTB/RIF AOR = 0.416, (95% CI: 0.236, 0.732). The diagnosis of MTB using Xpert MTB/RIF was associated with HIV status, P < 0.001 [Table 3]. Those living with HIV infection had a lower yield of MTB by Xpert MTB/RIF, odds ratio (OR) = 0.398 (95% CI: 0.266, 0.700); AOR = 0.556, (95% CI: 0.408, 0.758) compared to being HIV uninfected or having an unknown status. The MTB detection rate was highest in those with TB treatment failure (18.8%; 3/16). There were no significant differences in the MTB detection rate based on the TB status, P = 0.407 [Table 3].
| n=4526 (%) | Yes 584 (12.9%) | No 3942 (87.1%) | OR | 95% CI | AOR | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| Age group (years) | ||||||||
| <18 | 349 (7.7) | 24 (6.9) | 325 (93.1) | 0.398 | 0.266, 0.700 | 0.416 | 0.236, 0.732 | 0.002 |
| 18-30 | 877 (19.4) | 142 (16.2) | 735 (83.8) | 1.041 | 0.682, 1.589 | 1.161 | 0.759, 1.777 | 0.491 |
| 31-45 | 1385 (30.6) | 174 (12.6) | 1211 (87.4) | 0.774 | 0.511, 1.172 | 0.875 | 0.576, 1.328 | 0.530 |
| 46-60 | 1092 (24.1) | 144 (13.2) | 948 (86.8) | 0.818 | 0.537, 1.247 | 0.902 | 0.590, 1.377 | 0.632 |
| 61-75 | 625 (13.8) | 69 (11.0) | 556 (89.0) | 0.423 | 1.057 | 0.678 | 0.428, 1.071 | 0.096 |
| >75 | 198 (4.4) | 31 (15.7) | 167 (84.3) | 1 | 1 | |||
| Sex | ||||||||
| Females | 2305 (50.5) | 285 ((12.4) | 2020 (87.6) | 0.909 | 0.764, 1.082 | 0.946 | 0.793, 1.130 | 0.542 |
| Males | 2262 (49.5) | 306 (13.5) | 1956 (86.5) | 1 | ||||
| HIV status | ||||||||
| Infected | 756 (16.7) | 62 (8.2) | 694 (91.8) | 0.588 | 0.434, 0.798 | 0.556 | 0.408, 0.758 | <0.001 |
| Uninfected | 2443 (54.0) | 347 (14.2) | 2096 (85.8) | 1.090 | 0.896,1.325 | 1.095 | 0.900, 1.322 | 0.366 |
| Unknown | 1327 (29.30 | 175 (13.2) | 1152 (86.8) | 1 | 1 | |||
| TB status* | ||||||||
| Treatment failure | 16 (0.4) | 3 (18.8) | 13 (81.2) | 1.559 | 0.443, 5.487 | 1.346 | 0.381, 4.755 | 0.644 |
| Relapse | 50 (1.1) | 8 (16.0) | 42 (84.0) | 1.287 | 0.040, 2.179 | 1.077 | 0.501, 2.318 | 0.849 |
| MDR-TB contact | 24 (0.5) | 1 (4.2) | 23 (95.8) | 0.294 | 0.273 | 0.037, 2.036 | 0.206 | |
| New cases | 4436 (98.0) | 572 (12.9) | 3864 (87.1) | 1 |
The detection rate of MTB increased from 5.6% in 2016 to 23.8% in 2019, P < 0.001 [Figure 2]. The overall proportion of RR-TB cases was 7.5% (95% CI: 5.50, 9.94). [Figure 3] shows that RR-TB cases detected declined from 28% in 2016 to 4.6% in 2019, P < 0.001. Bivariate analysis [Table 4] showed that age >45 years was significantly associated with RR-TB detection status with an OR = 1.968, (95% CI: 1.011, 3.830) and an AOR = 2.046, (95% CI: 1.046, 4.004).
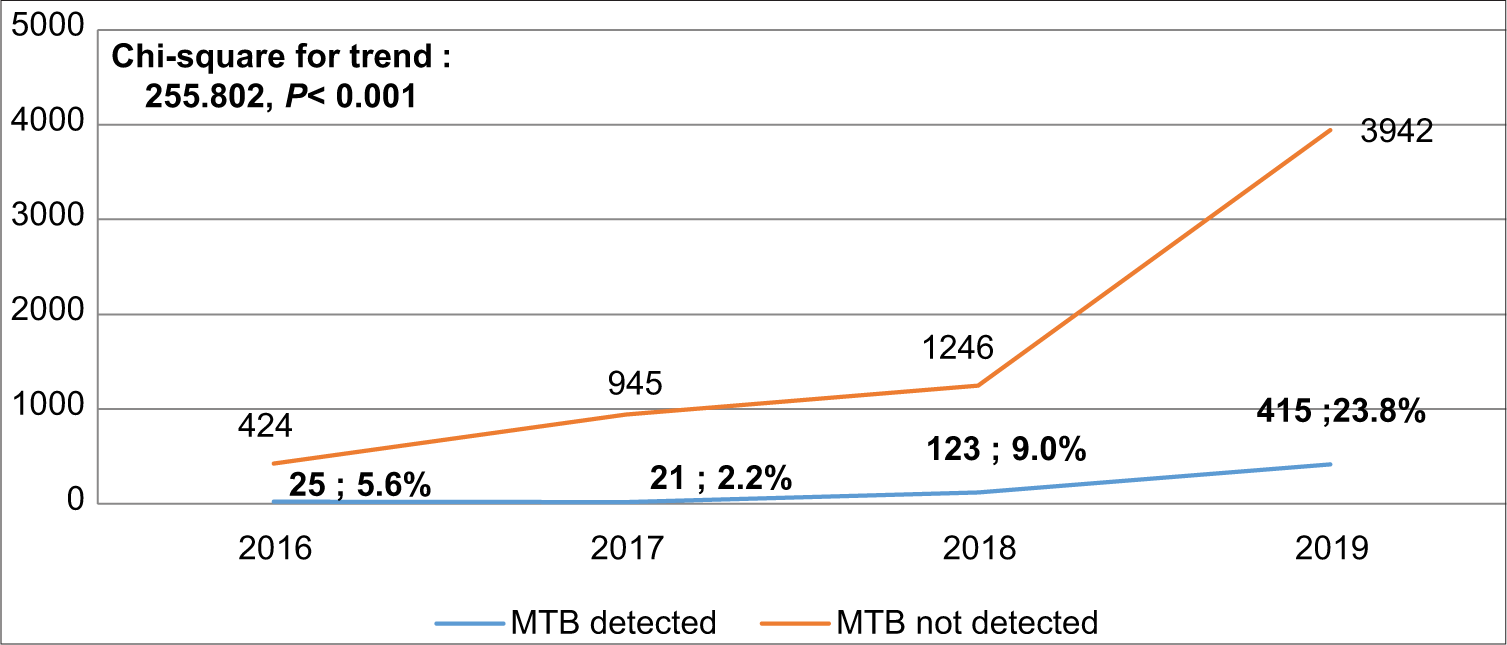
- Trend of prevalence Mycobacterium tuberculosis.
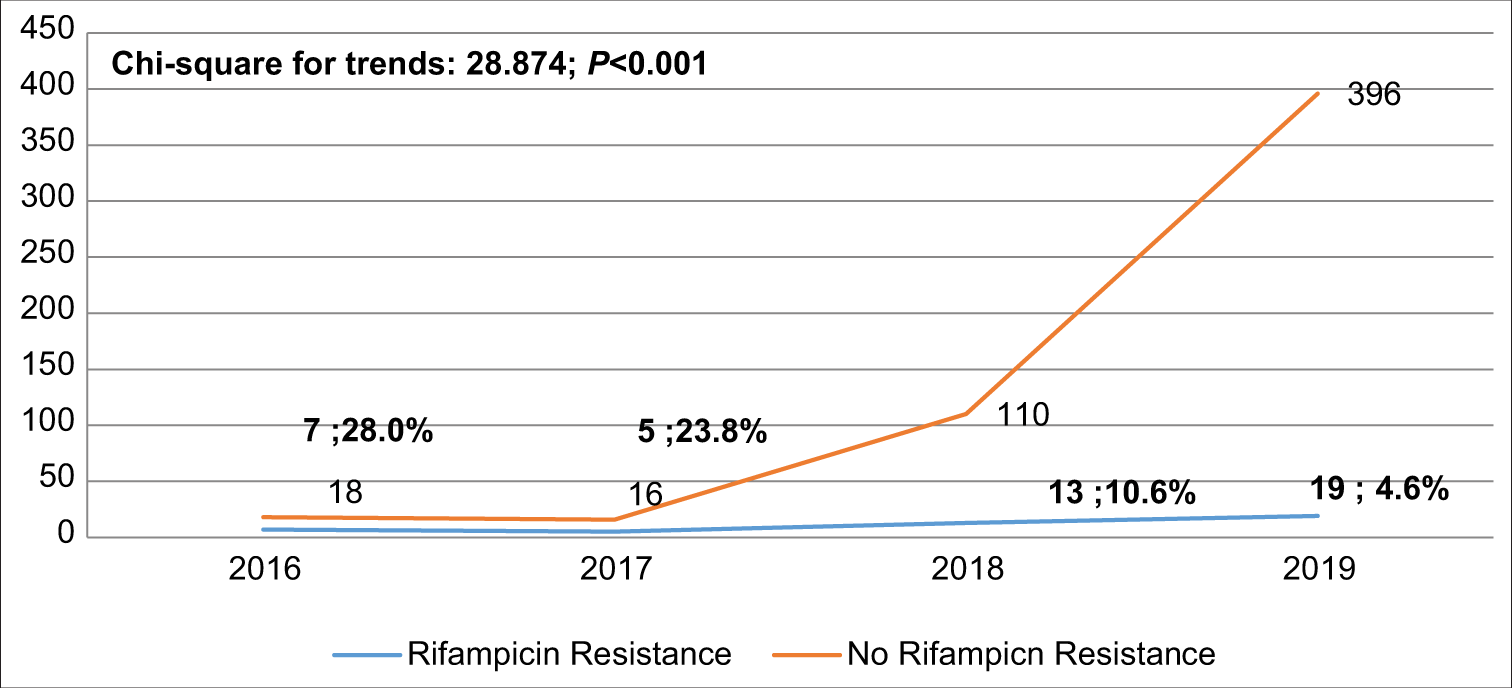
- Trend of rifampicin resistance among the confirmed Mycobacterium tuberculosis.
| Variables | Category | N (584) | Yes(44) | No (540) | OR | 95% CI | AOR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | ≤45 | 340 | 32 | 308 | 1 | 1 | |||
| >45 | 244 | 12 | 232 | 1.968 | 1.011, 3.830 | 2.046 | 1.046, 4.004 | 0.036 | |
| Sex | Male | 302 | 26 | 276 | 1 | 1 | |||
| Female | 282 | 18 | 264 | 1.470 | 0.804, 2.690 | 1.520 | 0.823, 2.807 | 0.181 | |
| HIV status | Unknown | 175 | 14 | 161 | 1 | 1 | |||
| Uninfected | 347 | 23 | 324 | 1.877 | 0.352, 2.181 | 0.897 | 0.356, 2.264 | 0.730 | |
| Infected | 62 | 7 | 55 | 0.891 | 0.457, 1.738 | 0.889 | 0.455, 1.736 | 0.819 | |
| TB status | New cases | 572 | 42 | 530 | 1 | 1 | |||
| Treatment failure | 3 | 1 | 2 | 6.310 | 0.561, 71.025 | 7.634 | 0.970, 60.070 | 0.053 | |
| Relapse | 8 | 1 | 7 | 1.803 | 0.217, 15.000 | 1.796 | 0.239. 13.470 | 0.569 | |
| MDR-TB contact | 1 | 0 | 1 | 4.161 | 0.167, 103.709 | 0.000 | 0.000 | 0.998 |
TB: Tuberculosis, MDR-TB: Multidrug-resistant tuberculosis, HIV: Human immunodeficiency virus, OR: Unadjusted odds ratio, AOR: Adjusted odds ratio, 95% CI: 95% Confidence interval
Forty-two of the 44 cases of the RR-TB (42/572; 7.3%, [95% CI: 5.31, 9.75]) were new cases, with TB treatment failure and relapse cases accounting for 10.1% (2/11; 10.1%, [95% CI: 0.35, 42.5]) of all RR-TB cases (P < 0.01).
[Figure 4] captured the geographical distribution pattern of MTB and RR-TB detection. RR-TB appears to be more common in densely populated semi-urban areas than in rural areas. The RR-TB appears to be more prevalent in communities where non-indigenous migrants from other states in Nigeria with higher HIV infection rates have settled.[27]

- Map of the density and distribution of Mycobacterium tuberculosis detected by GeneXpert and the rifampicin-resistant tuberculosis.
DISCUSSION
Given the high prevalence of MTB and RR-TB in Nigeria, this study examined the trends in MTB and RR-TB detection at TB treatment centers in Ogbomosho Southwest Nigeria using the Xpert MTB/RIF assay. In a large cohort of 4625 patients with presumed TB, the TB detection rate using a rapid nucleic acid-based assay (Xpert MTB/RIF) was 12.9%. The proportion of MTB cases detected is lower compared to 18.9% in Nasarawa State,[28] North-Central Nigeria, 37.7% in Lagos,[29] Southwest Nigeria, 17% in Benue,[30] North-Central Nigeria, and 16% in Kebbi[31] Northwest Nigeria. Although the studies mentioned above were all hospital based at referral TB treatment centers, detection of MTB using the Xpert MTB/ RR was less likely in individuals living with HIV infection which is in contrast to the higher yield among TB-HIV coinfected individuals in Benue and Nasarawa states. This might be related to the higher prevalence of HIV infection in these areas and concomitantly increased TB prevalence in the population with improved detection in the context of the use of Xpert MTB/RIF as the primary diagnostic tool.
Notably, the observed TB detection in the present study was higher than 9% obtained a decade earlier in Ibadan,[32] Southwest Nigeria. The present report is also higher than the report from Port Harcourt (4.5%)[33] in South-South Nigeria. These may be due to the use of the Xpert MTB/RIF for TB detection in the present study, as opposed to acid-fast bacilli (AFB) with ZN staining in the previous studies in Ibadan and Port Harcourt, South-South Nigeria. The Xpert MTB/RIF is known to have a higher sensitivity than traditional AFB with ZN stain. Furthermore, there are differences in the timelines and study durations between the current review and the preceding studies, with the majority of earlier studies lasting only 1 year.[28,29,31,33] In addition, this may reflect regional differences in the burden of TB in the country, with densely populated areas such as Lagos state having a higher burden than semi-urban locations.
Studies from other countries, on the other hand, reported a significantly higher detection rate of MTB, with centers in Nepal, Ethiopia, and Iraq reporting 23.6%, 30.5%, and 40%, respectively.[34-36] The preponderance of new presumed TB cases from the general population in the present study may account for the lower proportion of MTB cases compared to the report from Nepal, in which the denominator was comprised of high risk, secondary level screening of treatment failure cases, HIV-infected patients, and patients with radiographic evidence of TB who were all smear negative for the ZN acid-fast stain technique.[37]
This study observed that the proportion of patients tested with Xpert MTB/RIF increased over the 4-year study period, as did the yield of MTB cases detected. This finding corroborates the global trend in case notifications of people diagnosed with TB, from 2016 with peak notification between 2017 and 2019.[2-5] The detection of MTB in Southeast Asia followed a similar pattern to that observed in Africa. However, the magnitude of increased detection and notification was significantly less pronounced in the Eastern Mediterranean region, and a decline was observed in the Western Pacific region of the world in 2017.[11] The increased MTB notification in African region may be a consequence of the increased access to Xpert MTB/RIF, a more sensitive diagnostic tool than the ZN acid-fast stain technique.[7] In addition, throughout the years, screening criteria have been modified to allow for the testing of more people for MTB utilizing the Xpert MTB/RIF approach.
Notification of DR-TB is one of the surrogate measures of the effectiveness of global TB control programs, with varying reports based on diagnostic and treatment coverage. The present study’s finding of 7.5% (95% CI: 5.50, 9.94) RR-TB notification compares favorably with the published national average but is higher than the global average reported by the WHO in the 2019 report.[2] Our finding is lower than the WHO reported incidence in India, North Thailand, China, and the Russian Federation. A rifampicin resistance rate of more than 3% in non-MDR-TB is a poor predictor of TB mitigation strategies.[2,3,38-40] This finding has implications for the unfolding of the COVID-19 pandemic, where lockdowns and restricted access to care could be a driving force for the rapid spread of RR-TB, especially in settings with limited resources.[11] Thus, the need for more pragmatic approaches, including ongoing surveillance for resistance cannot be overemphasize as the world strives to achieve the third Sustainable Development Goal 3 target of eradicating TB by 2030.[2]
The observed 7.3% (95% CI: 5.31, 9.75) RR-TB among new cases compares to the national average of 4.3% (95% CI: 3.2– 5.5) in 2019 and the reported average of 8.6% in Eswatini, which is located in the East of South Africa. However, the percentage is lower than the global and African regional averages, as well as the Southeast Asian and Western Pacific averages of 3.4%, 2.5%, 2.6%, and 4.6%, respectively. In the present study, the reported percentage of 10.1 (95% CI: 0.35, 42.5) RR-TB among previously treated patients is in tandem across all of the above-mentioned regions and the global average.[2]
Age over 45 is a significant risk factor for developing RR-TB as well as a poor predictor of treatment outcome.[41] According to research from Africa, China, and Thailand, being over 45 years old is associated with a lower treatment success rate.[42-44] Older age was associated with higher comorbidity with increased pill burden and adverse events and poor treatment adherence. This age group is also predisposed to interact with MDR-TB patients, and has a higher risk of smoking, alcoholism, loss of follow-up, and other social factors that contribute to the development of resistant TB. In this subpopulation, a targeted approach different from DOTS may be more relevant.[45]
A reducing trend of RR-TB detection among confirmed MTB cases was observed over the study period. This is consistent with the trends of the global burden of MDR-TB between 1990 and 2017.[46] The declining RR-TB cases in this study could be credited to Nigeria’s ongoing effective National TB and HIV control programs, as well as the availability of effective anti-TB drugs. Our center also has an effective contact tracing protocol and effective adherence counseling, as well as a holistic approach to a tripartite nature of human existence that addresses the physical, mental, and spiritual needs of study participants, which has contributed to improved treatment adherence and trend observed.[46]
Strength and limitations
The study’s strength lies in its large dataset of 4625 presumptive TB cases analyzed with Xpert MTB/RIF over a 4-year study duration period. The retrospective and single-center nature of this study, on the other hand, may limit its generalizability. Finally, there was no TB culture or susceptibility testing in this study. For sensitivity testing, the PCR-based test has been shown to be accurate and can diagnose up to 75% of MDRTB which gives some credence to this study.
CONCLUSION
This study found an increase in MTB detection with Xpert MTB/RIF utilization. Ages >45 years have 2–4-fold increased risk of developing RR-TB and should be targeted for drug resistance prevention.
Data reference
This is available at Alao, Michael (2022), “Alao et al 2022. A 4 year data on GeneXpert MTB/RIF for MTB, rifampicin resistance, and TB HIV coinfection in a tertiary hospital in Nigeria. Mendeley Data, V1, doi: 10.17632/vhw6t2pdrg.[26]
Acknowledgment
We appreciate all the personnel at the TB treatment centers as well as the scientists at the NTBLCP affiliated TB laboratory in Ogbomosho who analyzed the samples. Thank you very much to Major General Allan Kigbu, Department of Paediatrics, Army Command, and NAOWA Hospital, Abuja, for reading the manuscript and providing valuable feedback. Most importantly, we wish to thank the patients who submitted the samples for the test.
Availability of data and materials
This is available at Alao, Michael (2022), “Alao et al 2022. A 4 year data on GeneXpert MTB/RIF for MTB, rifampicin resistance, and TB HIV coinfection in a tertiary hospital in Nigeria. Mendeley Data, V1, doi: 10.17632/vhw6t2pdrg. 10
Ethical approval
This study was a retrospective study, ethical approval was obtained from the Health Research Ethical Committee of the Bowen University Teaching Hospital with approval number BUTH/REC/-047.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Tuberculosis: Fact Sheet. 2021. Geneva: World Health Organization; Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis [Last accessed on 2022 Apr 21]
- [Google Scholar]
- Global Tuberculosis Report 2019 Geneva, Switzerland: World Health Organization; 2019.
- [Google Scholar]
- World Health Organization Global Tuberculosis Report 2020 Geneva: World Health Organization; 2020. p. :232.
- [Google Scholar]
- Global Tuberculosis Report 2020: Executive Summary. Geneva: World Health Organization; 2020.
- [Google Scholar]
- Global tuberculosis report 2020-reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;113:S7-12.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory diagnosis of tuberculosis in resource-poor countries: Challenges and opportunities. Clin Microbiol Rev. 2011;24:314-50.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. J Clin Tuberc Other Mycobact Dis. 2016;4:33-43.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of genexpert MTB/RIF assay in comparison to conventional drug susceptibility testing method for the diagnosis of multidrug-resistant tuberculosis. PLoS One. 2017;12:e0169798.
- [CrossRef] [PubMed] [Google Scholar]
- Methods used by WHO to Estimate the Global Burden of TB Disease In: Global TB Programme. Geneva: World Health Organization; 2016.
- [Google Scholar]
- Molecular Assays Intended as Initial Tests for the Diagnosis of Pulmonary and Extrapulmonary TB and Rifampicin Resistance in Adults and Children: Rapid Communication Geneva: World Health Organization; 2020.
- [Google Scholar]
- WHO Global Lists of High Burden Countries for Tuberculosis (TB), TB/HIV and Multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021-2025 In: Background Document. Geneva: World Health Organization; 2021.
- [Google Scholar]
- Prevalence of drug-resistant tuberculosis in Nigeria: A systematic review and meta-analysis. PLoS one. 2017;12:e0180996.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and psychosocial determinants of patients with tuberculosis/human immunodeficiency virus co-infection: A structural equation model approach. Niger J Clin Pract. 2022;25:105-9.
- [CrossRef] [PubMed] [Google Scholar]
- The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance In: Anti-tuberculosis Drug Resistance in the World. Report. Geneva: World Health Organization; 2000.
- [Google Scholar]
- Multidrug-resistant and extensively drug-resistant tuberculosis: A review. Curr Opin Infect Dis. 2008;21:587-95.
- [CrossRef] [PubMed] [Google Scholar]
- Barriers to reaching the targets for tuberculosis control: Multidrug-resistant tuberculosis. Bull World Health Organ. 2007;85:387-90.
- [CrossRef] [PubMed] [Google Scholar]
- Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: International multicentre randomised trial. Lancet. 2004;364:1244-51.
- [CrossRef] [PubMed] [Google Scholar]
- Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS One. 2009;4:e5561.
- [CrossRef] [PubMed] [Google Scholar]
- National Tuberculosis, Leprosy, and Buruli Ulcer Control Programme 2019. Annual TB Report. Nigeria: Federal Ministry of Health; Available from: https://www.health.gov.ng/doc/Draft-2019-NTBLCP-annual-report-22032020.pdf [Last accessed on 2022 Nov 01]
- [Google Scholar]
- Knowledge of international standards for tuberculosis care among private non-NTP providers in Lagos, Nigeria: A cross-sectional study. Trop Med Infect Dis. 2022;7:192.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of presentation of drug-resistant tuberculosis in Nigeria: A retrospective file review. Microbiol Res. 2022;13:609-19.
- [CrossRef] [Google Scholar]
- Oyo Population 2022 (Demographics Maps Graphs) Avaialable from https://www.oyopopulation2022(demographics,maps,graphs)worldpopulationreview.comworld-cities/oyo-population [Last accessed on 2022 Sep 01]
- [Google Scholar]
- Evaluation of the cepheid genexpert® system for detecting Bacillus anthracis. J Appl Microbiol. 2006;100:1011-6.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment outcomes of Nigerian patients with tuberculosis: A retrospective 25-year review in a regional medical center. PLoS One. 2020;15:e0239225.
- [CrossRef] [PubMed] [Google Scholar]
- "Alao et al 2022 Trends in MTB Rifampicin Resistance in a tertiary Hospital in Nigeria" Mendeley Data. 2022;V1
- [CrossRef] [Google Scholar]
- Rifampicin resistant Mycobacterium tuberculosis in Nasarawa State, Nigeria. Niger J Basic Appl Sci. 2017;14:21.
- [CrossRef] [Google Scholar]
- HIV epidemiology in Nigeria. Saudi J Biol Sci. 2018;25:697-703.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of rifampicin resistant tuberculosis and associated factors among presumptive tuberculosis patients in a secondary referral hospital in Lagos Nigeria. Afr Health Sci. 2018;18:472-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of rifampicin resistance tuberculosis among HIV/TB coinfected patients in Benue State, Nigeria. Pan Afr Med J. 2021;38:203.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of rifampicin-resistance presumptive M. tuberculosis cases among outpatients in Kebbi State, Nigeria. Afr J Infect Dis. 2021;15:47-52.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of clinical isolates of Mycobacterium tuberculosis at Ibadan, Nigeria. Niger J Physiol Sci. 2010;25:135-8.
- [Google Scholar]
- Multi-drug resistant Mycobacterium tuberculosis in port harcourt, Nigeria. Afr J Lab Med. 2018;7:805.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of tuberculosis from smear-negative presumptive TB cases using Xpert MTB/Rif assay: A cross-sectional study from Nepal. BMC Infect Dis. 2019;19:1090.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Mycobacterium tuberculosis infection and rifampicin resistance among presumptive tuberculosis cases visiting tuberculosis clinic of Adare General Hospital, Southern Ethiopia. SAGE Open Med. 2021;9:20503121211045541.
- [CrossRef] [PubMed] [Google Scholar]
- GeneXpert MTB/RIF assay-a major milestone for diagnosing Mycobacterium tuberculosis and rifampicin-resistant cases in pulmonary and extrapulmonary specimens. Med J Babylon. 2019;16:296.
- [CrossRef] [Google Scholar]
- Factors that influence current tuberculosis epidemiology. Eur Spine J. 2013;22:539-48.
- [CrossRef] [PubMed] [Google Scholar]
- The global tuberculosis epidemic and progress in care, prevention, and research: An overview in year 3 of the end TB era. Lancet Respir Med. 2018;6:299-314.
- [CrossRef] [PubMed] [Google Scholar]
- Trends and characteristics of drug-resistant tuberculosis in rural Shandong, China. Int J Infect Dis. 2017;65:8-14.
- [CrossRef] [PubMed] [Google Scholar]
- Drug resistance profiles and trends in drug-resistant tuberculosis at a major hospital in Guizhou Province of China. Infect Drug Resist. 2019;12:211-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of rifampicin-resistant tuberculosis among patients previously treated for pulmonary tuberculosis in North-Western, Nigeria. Niger J Med. 2017;58:161-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for poor treatment outcomes in patients with MDR-TB and XDRTB in China: Retrospective multi-center investigation. PLoS One. 2013;8:e82943.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with poor treatment outcome of tuberculosis in Debre Tabor, Northwest Ethiopia. BMC Res Notes. 2018;11:25.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and associated risk factors of drug-resistant tuberculosis in Thailand: Results from the fifth national anti-tuberculosis drug resistance survey. Trop Med Int Health. 2021;26:45-53.
- [CrossRef] [PubMed] [Google Scholar]
- Factors affecting therapeutic compliance: A review from the patient's perspective. Ther Clin Risk Manag. 2008;4:269.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in burden of multidrug-resistant tuberculosis in countries, regions, and worldwide from 1990 to 2017: Results from the global burden of disease study. Infect Dis Poverty. 2021;10:24.
- [CrossRef] [PubMed] [Google Scholar]






