Translate this page into:
Aspergillus mediastinal lymphadenopathy with bronchial invasion mimicking cancer in an immunocompetent host
*Corresponding author: Shriram S. Shenoy, Department of Pulmonology, Manipal Hospital Bangalore, Bengaluru, Karnataka, India. drshriramshenoy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shenoy SS, Sachin D, Sundaram P. Aspergillus mediastinal lymphadenopathy with bronchial invasion mimicking cancer in an immunocompetent host. J Pan Afr Thorac Soc 2020;1(1):42-5.
Abstract
The clinical presentation of pulmonary mycotic disease is determined by the interaction between the fungus and host. Inhalation of spores resulting in sino-pulmonary disease is the most frequent manifestation. It can have a myriad radiological manifestations ranging from ground glassing, fleeting consolidations, and cavitation to interstitial thickening. Mediastinal lymphadenopathy locally eroding into multiple bronchi is highly unusual. The main clinical presentations are cough, hemoptysis, and dull chest pain. Diagnosis is based on either indirect serological tests or direct visualization of hyphae under the microscope. We present a case of a 25-year-old laborer with hemoptysis and weight loss; found to have mediastinal lymphadenopathy invading endobronchially. Tuberculosis, lymphoma, and bronchogenic carcinoma were our initial differential diagnosis, but invasive aspergillosis was later confirmed following biopsy. The role of a fungal etiology in pulmonary lesions among immunocompetent individuals is often overlooked. This case highlights the need to keep a broad differential in mind while dealing with mediastinal lymphadenopathy.
Keywords
Aspergillus
Bronchoscopy
Lymph node
Invasive
INTRODUCTION
The clinical presentation of pulmonary mycotic disease is determined by the interaction between the fungus and host. According to Pilaniya et al., highest risk of the development of disease is in those with impaired cell mediated immunity such as HIV/AIDS, combined variable immunodeficiency syndrome, and organ transplant recipients. The presence of invasive disease, either locally or disseminated, is highly unusual in young, non-immunodeficient persons. The mere presence of old age per se is not a strong predisposing factor for invasive disease; unless complicated by diabetes or treatment for any malignancy. Inhalation of spores resulting in sino-pulmonary disease is the most frequent manifestation in the general population.[1] Disease spectrum ranges from fungal sinusitis, allergic bronchopulmonary mycosis, severe asthma with fungal sensitization to pneumonia, hematogenous disseminated mycosis, and fibrosing chronic pulmonary mycosis. Invasive aspergillosis has also been diagnosed in immunocompetent hosts after massive exposure to fungal spores.[2] This is related to activities such as cave exploration, mining, and construction work. The main manifestations are chronic weight loss with cough, hemoptysis, and dull chest pain.[3] Thus, differentiating fungal disease from tuberculosis or malignancy becomes extremely important particularly in immune-competent individuals.
CASE REPORT
We present a case of a 25-year-old immunocompetent laborer (involved in extraction and transportation of mud, sand, and stone to various construction sites), resident of a coal mining village in Northern India; who presented with dry cough, hemoptysis, weight loss, and low grade fever since 3 months. He had no known comorbidities or history of any medications. He was a non-smoker. There was no significant family/contact history of tuberculosis. His chest X-ray done at a local clinic showed mediastinal widening [Figure 1]. He was started on empirical treatment for tuberculosis to which he was not responding. In our hospital, a computed tomography (CT) of the chest done showed bilateral mediastinal lymphadenopathy eroding endobronchially into both lobar bronchi [Figure 2]. Bronchoscopy revealed fungating exophytic masses with erosion of the bronchial wall, in the left apicoposterior segment, left superior basal segment, and right anterior basal segment. Endobronchial forceps biopsy, protected brush specimen, and saline washings were taken [Figure 3].
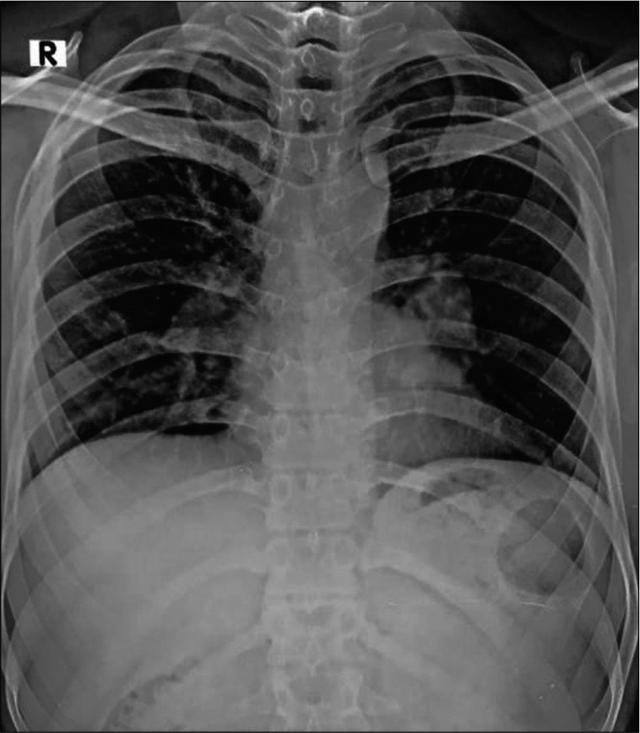
- Chest X-ray showing mediastinal widening.
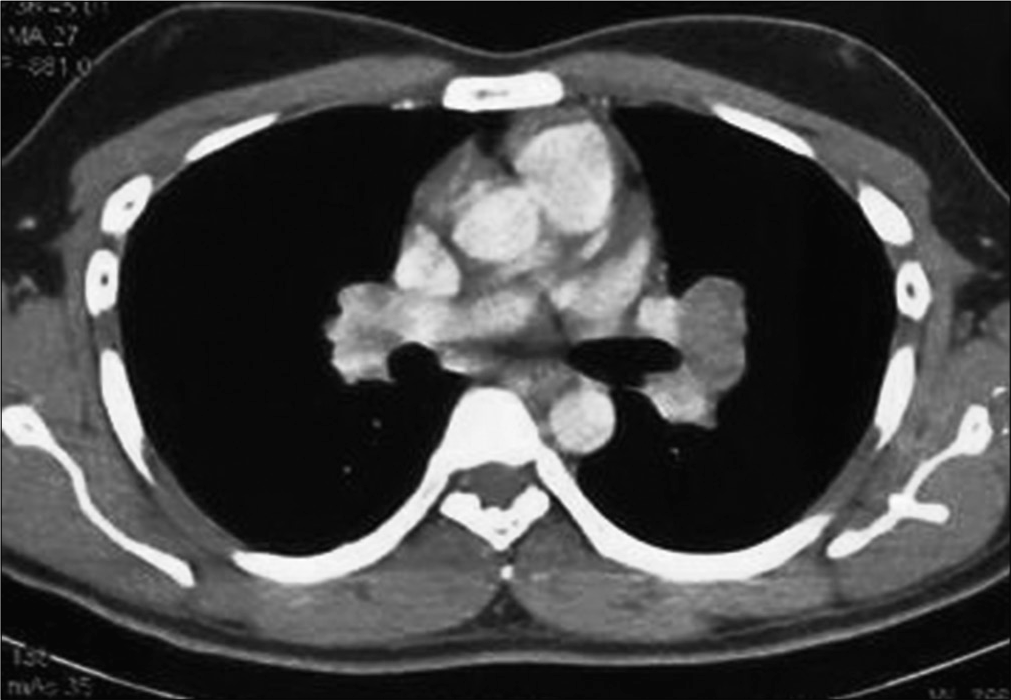
- Computed tomography showing mediastinal lymph nodes invading the airway lumen.
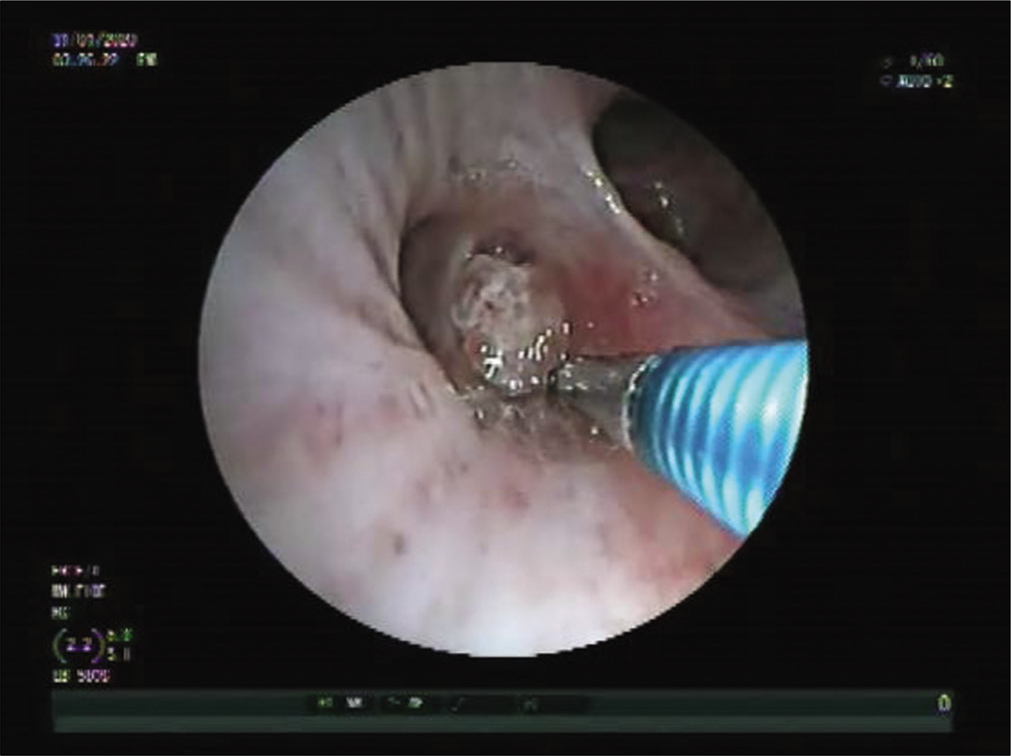
- Bronchoscopic image showing exophytic mass like lesions.
Samples were negative for molecular tests for tuberculosis; aerobic cultures did not grow any organism. Gram stain and Ziehl–Neelsen stain were negative. KOH mount showed branching septate hyphae suggestive of Aspergillus species [Figure 4]. Histopathology revealed septated hyphae branching at acute angles with typical “aspergillus conidia” seen on periodic acid stain [Figure 4b].
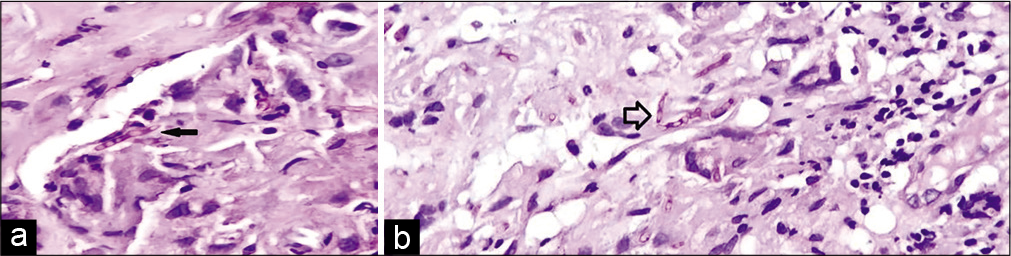
- (a) Typical Aspergillus conidia seen on microscopy (black arrow). (b): Aspergillus septated hyphae branching at acute angles (hollow arrow).
He was started on induction therapy with Voriconazole for 2 weeks, followed by liposomal amphotericin B with regular monitoring of renal function and liver enzymes. At 2 months of follow-up, he has significant improvement, gained 6 lbs. of weight and is tolerating therapy.
DISCUSSION
Many fungi have been identified as pathogenic, of which Candida, Aspergillus, and Cryptococcus are the most common.[4] Mediastinal masses resulting from Aspergillosis are extremely rare and occur in few severely immunosuppressed patients.[5] Pulmonary Aspergillus can manifest as a spectrum from colonization and asthma to fungal ball to invasive pulmonary aspergillosis (IPA).[6] The occurrence of IPA in a previously healthy subject is highly unusual. It is seen mainly in patients with congenital or acquired defects in immunity such as HIV/AIDS, combined variable immunodeficiency syndrome, anticancer therapy, and organ transplant.[7] Differential diagnosis may include primary or secondary carcinoma, tuberculosis, silicosis, or sarcoidosis.[8]
Reichenberger et al. described infiltrates and nodularity in IPA which are nonspecific, representing very early consolidation. Appearance of a hemorrhagic lesion termed “halo sign” is typical for IPA. It is the result of coagulative necrosis with hemorrhagic infarction. It is present over a short period of 7–15 days after onset of IPA and has differentials such as alveolar hemorrhage, bronchiolitis obliterans pneumonia, viral infections, Kaposi sarcoma, and granulomatosis with polyangiitis. The “air crescent” sign may also be seen in few cases of IPA. Most of these findings have been described in neutropenic hosts.[8] In our patient, the lung parenchyma was largely normal. The fungal etiology only involved the mediastinal lymph nodes and the airways. This, we believe is extremely rare. In immunocompetent hosts, about 90% of blood cultures are negative adding to the diagnostic challenge.[9] Galactomannan (GM) is a part of Aspergillus species cell membrane and is released when it invades the vessels. According to one study, the sensitivity, specificity, and negative predictive value for a bronchoalveolar lavage GM level of more than 1.0 were 100%, 88.1%, and 100%, respectively.[10] Given the high likelihood of false positive results, bronchoalveloar fluid GM test should not be ordered routinely in this patient population.[11]
In rare cases, IPA develops in patients with other conditions, such as bacterial pneumonia, chronic obstructive pulmonary disease, liver failure, sinusitis, or after surgery.[12] Our patient did not have any of these comorbidities. We suspect maybe poor socio-economic status resulting in poor nutrition combined with lack of safety equipment at site of construction may have predisposed him to develop disease.
Antifungal susceptibility of Aspergillus is reserved for patients suspected to have an azole resistance or for epidemiological purposes.[13] However, early initiation of antifungal therapy in strongly suspected IPA has shown to retard progression and is recommended due to limited performance of diagnostic testing.[14] The first pivotal treatment trial performed for IPA demonstrated better survival in patients on voriconazole compared with Amphotericin as initial treatment. Since that trial, multiple cohort studies supported this recommendation. Thus, for primary treatment of IPA in adults, intravenous or oral voriconazole is recommended for most patients.[15]
However, few prominent studies have also proven benefits of initial Amphotericin B.[15] Recently, a randomized trial compared voriconazole with isavuconazole, which demonstrated that fewer drug related adverse effects in the isavuconazole group.[16] Based on this, isavuconazole was Food and Drug Administration approved for first-line therapy and is recommended as an alternative primary therapy for IPA.[17]
Liposomal Amphotericin B usually given at 5 mg/kg/ day has been reported to be effective particularly as a salvage therapy.[18] Preliminary data suggest potential benefits for combination therapy with voriconazole with anidulafungin.[19] While caspofungin has shown benefit in non-comparative studies, its use as monotherapy is not currently recommended.[20]
Therapeutic monitoring includes evaluation of all symptoms and signs and radiographic imaging at regular intervals. The frequency with which a CT should be performed should be individualized on the basis of severity of illness. Kousha et al. reported that the use of CT scan early in the course of invasive aspergillosis leads to better outcomes.[21]
In our patient, a CT was done early on presentation, followed by bronchoscopy and on diagnosis; we started induction therapy with Voriconazole 200 mg twice a day with significant clinical improvement.
CONCLUSION
The presentation of fungal erosive disease is possible irrespective of immune status particularly in cases of potential exposure to fungi.
We suggest considering fungal etiology as a differential diagnosis in the evaluation of patients with mediastinal lymphadenopathy irrespective of immune status. This approach provides opportunity for the diagnosis of a potentially treatable condition and the institution of appropriate therapy.
Acknowledgment
We humbly dedicate this article to our patients, to whom we are eternally duty-bound.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:e1-60.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270-7.
- [CrossRef] [PubMed] [Google Scholar]
- Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419-31.
- [CrossRef] [PubMed] [Google Scholar]
- Bulky mediastinal aspergillosis mimicking cancer in an immunocompetent patient. Ann thorac Surg. 2014;98:1472-5.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of patients with chronic pulmonary aspergillosis according to the new ESCMID/ERS/ECMM and IDSA guidelines. Mycoses. 2017;60:136-42.
- [CrossRef] [PubMed] [Google Scholar]
- Acute invasive pulmonary aspergillosis, shortly after occupational exposure to polluted muddy water, in a previously healthy subject. J Bras Pneumol. 2015;41:473-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19:743-55.
- [CrossRef] [PubMed] [Google Scholar]
- Aspergillus endocarditis after open heart surgery: An epidemiological investigation. J Hosp Infect. 1990;15:245-53.
- [CrossRef] [Google Scholar]
- Use of bronchoalveolar lavage to detect galactomannan for diagnosis of pulmonary aspergillosis among nonimmunocompromised hosts. J Clin Microbiol. 2007;45:2787-92.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary aspergillosis: Clinical presentation, diagnostic tests, management and complications. Curr Opin Pulm Med. 2010;16:242-50.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility testing in Aspergillus species complex. Clin Microbiol Infect. 2014;20:49-53.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging findings in acute invasive pulmonary aspergillosis: Clinical significance of the halo sign. Clin Infect Dis. 2007;44:373-9.
- [CrossRef] [PubMed] [Google Scholar]
- Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408-15.
- [CrossRef] [PubMed] [Google Scholar]
- Liposomal amphotericin B as initial therapy for invasive mold infection: A randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad Trial) Clin Infect Dis. 2007;44:1289-97.
- [CrossRef] [PubMed] [Google Scholar]
- Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760-9.
- [CrossRef] [Google Scholar]
- Effectiveness of amphotericin B lipid complex (ABLC) treatment in allogeneic hematopoietic cell transplant (HCT) recipients with invasive aspergillosis (IA) Bone Marrow Transplant. 2005;36:873-7.
- [CrossRef] [PubMed] [Google Scholar]
- Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann Intern Med. 2015;162:81-9.
- [CrossRef] [PubMed] [Google Scholar]
- Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: An European organization for research and treatment of cancer study. Bone Marrow Transplant. 2010;45:1227-33.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary aspergillosis: A clinical review. Eur Respir Rev. 2011;20:156-74.
- [CrossRef] [PubMed] [Google Scholar]






