Translate this page into:
Influenza, malaria parasitemia, and typhoid fever coinfection in children: Seroepidemiological investigation in four Health-care Centers in Lagos, Nigeria
*Corresponding author: Abdul-Azeez Adeyemi Anjorin, Department of Microbiology (Virology Research), Lagos State University, Ojo, Lagos, Nigeria. abdul-azeez.anjorin@lasu.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Anjorin AA, Babalola SR, Iyiade OP. Influenza, malaria parasitemia, and typhoid fever coinfection in children: Seroepidemiological investigation in four Health-care Centers in Lagos, Nigeria. J Pan Afr Thorac Soc 2020;1(1):26-34.
Abstract
Objectives:
There are similarities in the presentation of influenza-A infection, malaria, and typhoid fever which include their overlapping clinical symptoms such as fever and myalgia. Coinfection may be easily missed and may lead to more severe associated morbidity. This study, therefore, investigated the prevalence of coinfection of influenza A, malaria, and typhoid fever in children in four locations in Lagos and determined their age, gender, and location-related prevalence.
Materials and Methods:
A cross-sectional hospital-based study was conducted between March and October 2018. Children less than 15 years attending four health centers in Festac, Amuwo, Ojo, and Shibiri were recruited consecutively. Demographic and epidemiological data were obtained using structured questionnaires, to ascertain children with influenza-like symptoms. Their blood samples were then tested with rapid diagnostic method for malaria and typhoid fever. The children were further screened for influenza-A-specific IgM using ELISA method. Descriptive statistics were reported while p-values were determined for comparable parameters using Chi-square.
Results:
There were 364 children aged <1–14 years including 207 (56.9%) males. Of the 364 children tested, 76/364 (20.9%) were seropositive for influenza-A virus out of which 47/76 (61.8%) had malaria parasitemia, 42/76 (55.3%) had typhoid fever, and 21/76 (27.6%) were coinfected with both malaria parasites and Salmonella enteric Typhi. Children coinfected with influenza-A and malaria were found with a higher frequency of chest pain and cold/chill symptom respectively compared to children having influenza alone (P = 0.0001). Seropositivity for influenza was recorded in all the months studied with the month of March recording the highest influenza-A seropositivity of 20/76 (26.3%) (P = 0.02).
Conclusion:
This study detected 27.6% trio coinfection seroprevalence of influenza Type-A, malaria, and typhoid fever among children population. The finding is unique being the first of such report, to the best of our knowledge. Children coinfected with influenza-A and malaria had greater morbidity.
Keywords
Influenza virus
Malaria parasitemia
Typhoid fever
Seroepidemiology
Nigeria
INTRODUCTION
Influenza, an infectious respiratory disease caused by enveloped RNA virus of the family Orthomyxoviridae, is largely spread through aerosols and droplets generated through coughing and sneezing.[1,2] Annually, influenza virus infects about 1 billion individuals with resultant 3–5 million severe cases and 290,000–650,000 deaths globally.[3,4] It affects individuals of all ages including children, especially those with underlying health conditions.[5,6] Children account for 20–30% of the annual infection with those from developing countries accounting for 99% of deaths due to influenza-related infections.[4] Over 250,000 African children, <5 years are hospitalized with influenza and have annual hospitalization rate 3 times higher than the children in developed countries.[7] The clinical prognosis of influenza ranges from mild upper respiratory tract infection to acute and chronic diseases. It is generally characterized with rapid onset of fever, malaise, sore throat, cough, runny nose, and body pain or with severe illness, hospitalization, and death particularly in high-risk population including children.[8] In Africa, studies have reported influenza infection in pregnancy, and in patients with HIV, sickle cell anemia, and tuberculosis with prevalence of 48%, 3.9%, 2.0%, and 10%, respectively.[9-12] Other studies have also shown coinfection of influenza with asthma, pneumonia, and malaria.[13,14] There is, however, a dearth of information on coinfection of influenza virus with typhoid fever, and malaria parasitemia, hence the importance of this study, as it will throw more light on its existence and clinical implications of such coexistence. Unfortunately, in Nigeria, there is no national policy on influenza vaccination against this vaccine preventable disease either in the general population or among the high-risk group including children and pregnant women.
Malaria is a parasitic disease caused by a single-celled protozoan of the genus Plasmodium belonging to the phylum Apicomplexa.[15] It is spread from person to person through the bite of infected female Anopheles mosquito.[16] Out of the 3.2 billion people at risk of malaria infection, about 214 million cases occur yearly particularly in Africa with Nigeria having the highest number of cases.[17,18] It leads to 438,000 deaths with sub-Saharan Africa accounting for 90%,[16] of which more than two-third occur in children <5 years old.[19] In Nigeria, malaria accounts for 60% of outpatient visits, 30% child mortality, and 11% of maternal mortality.[20] In general, children with malaria often present with fever, chills, headache, myalgia, and vomiting which are similar to some of the influenza symptoms.[21]
Typhoid fever is a systemic infection caused by Gram-negative bacterium Salmonella enteric Typhi (S. Typhi) which is transmitted from person to person through fecal–oral route.[22] In some areas, the incidence rate occurs among children below 5 years.[23] However, it was estimated in 2010 that typhoid fever caused 26.9 million cases with 217,000 deaths globally.[24] Symptoms include fever, diarrhea, loss of appetite, and abdominal cramp.[25] Typhoid fever is a significant health problem, particularly among children and adolescent in developing countries[26] as a result of poor sanitation and unsafe food and water supply usually contaminated with human feces.[27]
Children in sub-Saharan Africa including Nigeria are vulnerable to influenza, malaria, and typhoid coinfection with little or no information on their coexistence. Therefore, there is a need to: (a) Investigate the etiologies of overlapping clinical presentations and the possibility of coinfection of influenza with malaria and typhoid fever and (b) provide data on the likely clinical burden of the coinfection, along with age distribution, gender, and location of the children. Hence, this study was aimed to determine the seroprevalence of influenza and its coinfection with malaria and typhoid fever in the children attending health centers in Lagos, Nigeria.
MATERIALS AND METHODS
Study design, site, and subjects
This research was a cross-sectional hospital-based study in which both inpatients and outpatients were recruited from government/public health institutions by purposive sampling in four different locations: Festac (maternal and child health), and three primary health centers (PHCs) of Amuwo, Ojo, and Shibiri in Lagos State. The PHCs were chosen based on different local council development areas, availability of screening facilities for routine malaria and typhoid fever tests, and the population of nursing mothers attending such centers for pediatric treatment. The maternal and child center is a reference pediatric center for the treatment of infants and children. It also provides prenatal, postnatal, family planning, and other medical services to mothers. The concept of the study was explained to their parent/ guardian and their consent sought with verbal agreement and written documentation. Following signed informed consent, information on demographic and epidemiological parameters including age, gender, sampling location, comorbidity, and clinical signs and symptoms were collected using structured interviewer-administered questionnaires. Blood samples were collected from children of consenting parents, who were unvaccinated for influenza. Data were collected between the months of March and October 2018.
Study population
These were children <15 years old attending the outpatient clinics or were inpatients at the respective facilities, who had influenza-like illness including: fever above 38°C, rhinorrhea, and cough and who had positive tests for malaria parasite and Salmonella Typhi infection. The study excluded participants above 15 years or patients without fever and those that were negative for malaria parasite and S. Typhi infection. They were proportionately recruited consecutively until the sample attained the required calculated minimum sample size of 236 following Kish, Leslie formula of n = Z2p (1–p)/d2, where Z = reliability coefficient of 1.96 at 95% confidence interval. A presumed prevalence (p) of 18.9% was used in the sample size calculation, according to a previous study.[28]
Ethics
Ethical approval was obtained from the Health Research and Ethics Committee of Lagos State University Teaching Hospital Research and Ethics Committee (LREC) with reference number: LREC.06/10/1030 while each hospital/ center permission was sought from the head of the health center/hospital where the samples were collected.
Sample collection and preservation
Five milliliters of blood samples were drawn by the phlebotomists or midwives using sterile needle and syringe. Each blood sample was shared into labeled EDTA and plain container for malaria parasites/typhoid fever and influenza A viral assay respectively. Malaria parasites and S. Typhi samples were further processed at the health centers of collection while sample aliquots for influenza A viral assay were then kept in the coolers with ice packs (<4°C) before being transported to the laboratory within (30–60 min). Sera were separated from the blood by centrifugation at 3000 rpm for 10–15 min and transferred into labeled Cryovial tubes with the aid of Pasteur pipettes, before they were stored in the freezer at –30°C until ready-to-use for testing.
Laboratory procedure
Laboratory analysis was performed at the Virology Research Laboratory, Department of Microbiology, Lagos State University, Ojo. All the children were tested by rapid diagnostic test (RDT) for the detection of malaria histidine-rich protein II and Plasmodium lactate dehydrogenase (Accessbio or Medicon BioCheck, USA) and S. Typhi for typhoid fever (CTK Biotech, USA or Omega, UK). They were further screened for the qualitative and quantitative determination of influenza A virus-specific IgM antibody using the ELISA method (Demeditec, Germany). The assay kits used had 100% clinical specificity and 100% sensitivity with no cross-reactivity to RSV, adenovirus, and parainfluenza 1/2/3.[29]
The RDT for detecting the malaria parasite and S. Typhi infection was performed at the health centers. The collected whole blood or plasma, as appropriately indicated by the manufacturer, was applied into the sample pad. Buffer solution (two drops) were added (when whole blood sample was used) into the test kit round hole. Results were recorded and interpreted after 15 min. The appearance of a line in both the control and test region of the testing kit was recorded as positive result. Any other variations of results were recorded as negative or invalid results. Invalid results were, however, repeated and those that became positive then treated as such. Enzyme-linked immunosorbent assay was performed as follows: Microtiter wells were labeled for the standards, controls, and samples as well as for substrate blank. The samples were diluted with ready-to-use sample diluents provided with the test kit in the ratio 1:100 (1 μl serum + 100 μl sample diluents). A 100 μl of the ready-to-use standard controls and diluted samples were pipetted into the microtiter wells leaving one well empty for the substrate blank. The microplate was covered with reusable plate cover and incubated at room temperature for 60 min. Unused reaction fluid was emptied and 300 μl of diluted washing solution was added. The wells were washed 3 times while remnant fluid was removed by gently tapping the microtiter plates on disposable papers. Ready-to-use enzyme conjugate (100 μl) was added into the wells leaving one well for the substrate blank. The microplates were covered with reusable plate cover and incubated at room temperature for 30 min. The plates were emptied and another 300 microlitres of diluted washing solution was added (3 times) for washing. Remnant fluid was again removed by tapping the plate gently on disposable papers. Ready-to-use substrate (100 μl) was added into the wells and the substrate blank. The plate was covered and incubated at room temperature in the dark for 20 min. A blue color was observed before the substrate reaction was terminated with 100 μl of ready-to-use stop solution. A yellow color developed while the absorbance of the wells was read with ELISA microplate reader (Emax precision, Molecular Devices, California, USA) at 450 nm wavelength. Each assay batch was performed with positive and negative controls. All the test results were expressed based on the standard curve provided with the assay kit supplied by the manufacturer. Samples were considered positive if the antibody concentration was >1.148 U/mL based on cutoff specification of the manufacturer. A positive test result to specific IgM antibody to influenza A virus implies that the individual was recently infected with a circulating wild-type influenza A virus in the population and not vaccine strain, since influenza vaccination is not a practice in Nigeria.
Statistical analyses
Epidemiological data were systematically analyzed using GraphPad Prism Version 8.0.1 (244), San Diego, USA. P-values with statistically significant differences of P < 0.05, between epidemiological data including age, gender, and study locations, were measured using Chi-square.
RESULTS
A total of 364 children were included in the study including 207 (56.9%) males. Demographic data showed median age of 3 (mean 3.8, mode 2, range of <0.5–14) years. [Figure 1] shows the distribution of children recruited from each health center. Of the 364 children investigated for IgM antibody specific to influenza A virus, 20.9% (76/364) were seropositive of which 61.8% (n = 47/76) were male. Out of the 76 influenza A seropositive children, 61.8% (47/76) were positive for malaria parasite, 55.3% (42/76) were positive for typhoid fever, and 27.6% (21/76) had coinfection of both malaria parasitemia and typhoid fever [Figure 2].
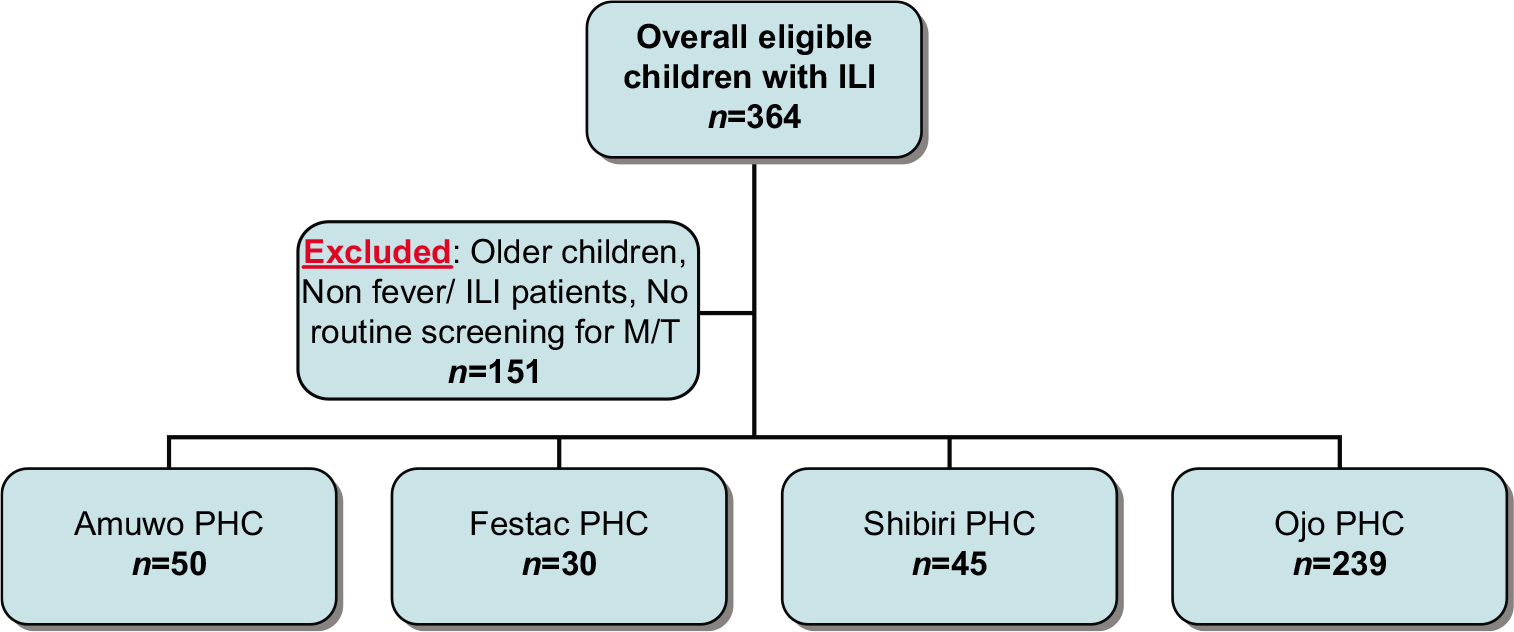
- Flow diagram for inclusion and sampling of children with influenza-like illness screened for malaria and typhoid fever. PHC: Primary health-care center, M: Malaria, T: Typhoid.
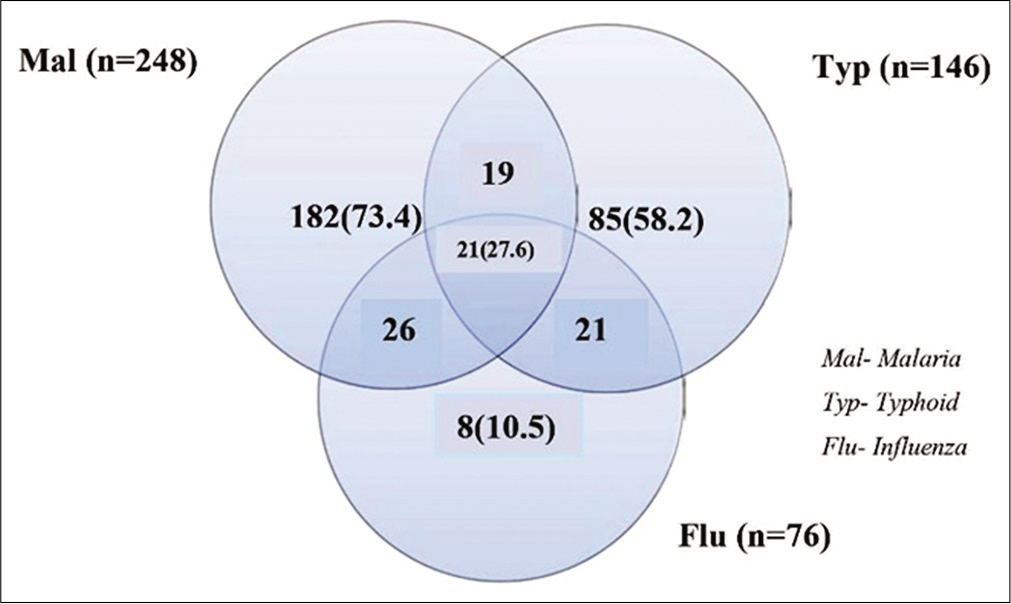
- Venn diagram showing the distribution (%) of influenza coinfection with malaria parasitemia and typhoid fever among health-care attendee children in Lagos, Nigeria.
[Table 1] shows the distribution of positive tests across age group, gender, health center, and comorbid conditions. Children in the age group of 1–4 years recorded the highest influenza A virus seropositivity of 55.3% (42/76) while those <0.5 year were the least exposed to influenza A virus with a prevalence of 4% (3/76), P < 0.001. Conversely, children of age group <0.5 year recorded the highest burden of influenza A coinfection with malaria parasitemia and typhoid fever 23.8% (n = 5) compared to influenza A monoinfection 4% (n = 3). A higher burden of influenza A with malaria parasitemia and influenza A with typhoid fever coinfections, 21.3% (n = 10) and 23.8% (n= 10), respectively, were detected in children aged 0.5–1 years compared to influenza A infection alone 9.2% (n = 7) [Table 1]. In addition, Ojo PHC had significant higher influenza A seropositivity 48.7% (n = 37) while the lowest, 13.2% (n = 10) seroprevalence was detected in Festac town location of Lagos State. None of the children with asthma was seropositive for influenza.
| Epidemiological data | Mal (%) | Typ (%) | Flu (%) | Flu+Mal (%) | Flu+Typ (%) | Flu+Mal+Typ (%) | P-value |
|---|---|---|---|---|---|---|---|
| (n=248) | (n=146) | (n=76) | (n=47) | (n=42) | (n=21) | ||
| Age range (years) | |||||||
| <0.5 | 17 (6.9) | 10 (6.8) | 3 (4) | 14 (29.8) | 6 (14.3) | 5 (23.8) | 0.0001 |
| 0.5–1 | 24 (9.7) | 16 (11) | 7 (9.2) | 10 (21.3) | 10 (23.8) | 3 (14.3) | |
| 1–4 | 130 (52.4) | 40 (27.4) | 42 (55.3) | 15 (31.9) | 21 (50) | 9 (42.9) | |
| 5–9 | 46 (18.5) | 51 (34.9) | 15 (19.7) | 5 (10.6) | 4 (9.5) | 4 (19) | |
| 12–14 | 31 (12.5) | 29 (19.9) | 9 (11.8) | 3 (6.4) | 1 (2.4) | 0 (0) | |
| Gender | |||||||
| Male | 136 (54.8) | 85 (58.2) | 47 (61.8) | 29 (61.7) | 30 (71.4) | 13 (61.9) | 0.43 |
| Female | 112 (45.2) | 61 (41.8) | 29 (38.2) | 18 (38.3) | 12 (28.6) | 8 (38.1) | |
| Location | |||||||
| Ojo | 171 (69) | 100 (68.5) | 37 (48.7) | 29 (61.7) | 22 (52.4) | 13 (61.9) | 0.04 |
| Shibiri | 30 (12.1) | 20 (13.7) | 15 (19.7) | 11 (23.4) | 8 (19) | 7 (33.3) | |
| Amuwo | 27 (10.9) | 12 (8.2) | 14 (18.4) | 4 (8.5) | 7 (16.7) | 1 (4.8) | |
| Festac | 20 (8) | 14 (9.6) | 10 (13.2) | 3 (6.4) | 5 (11.9) | 0 (0) | |
| Underlying health condition | |||||||
| Sickle cell | 10 (4) | 6 (4.1) | 2 (2.6) | 1 (2.1) | 0 (0) | 0 (0) | |
| Asthma | 0 (0) | 2 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Mal: Malaria, Typ: Typhoid, Flu: Influenza
[Figure 3] shows the clinical symptoms reported by study participants who were seropositive to influenza A virus and had coinfection with both malaria parasitemia and typhoid fever. The symptoms include fever, catarrh, cough, body weakness, sore throat, chest pain, and cold/chills. Although the most common of all the clinical symptoms recorded was fever of 48.6% in a high number of children (n = 71) having typhoid fever, this was keenly followed by fever of 47.6% in a small proportion of children (n = 10) having trio coinfection of influenza A, malaria parasitemia, and typhoid fever. Children (n = 7) with trio coinfection of influenza A, malaria parasitemia, and typhoid fever presented the highest 33.3% clinical symptom of catarrh compared to 26.3% (n = 20) in influenza monoinfection. In addition, children with coinfection of influenza A and malaria parasitemia accounted for a higher number of chest pain of 12.8% (n = 6) compared to chest pain complaint of 6.6% recorded in children (n = 5) having influenza A monoinfection (P = 0.0001).

- Distribution of clinical symptoms among children having coinfection of influenza, malaria parasitemia, and typhoid fever in Lagos, Nigeria. I: Influenza, M: Malaria, T: Typhoid.
Seropositivity to influenza A virus was recorded on all months across the duration of the study but the month of March accounted for the highest seroprevalence of 26.3% (20/76) and 42.9% (9/21) to influenza A monoinfection, and trio coinfection of influenza A, malaria parasitemia, and typhoid fever, respectively [Figure 4].

- Distribution of influenza, malaria parasitemia, and typhoid fever coinfection in children from March to October in Lagos, Nigeria. M: Malaria, T: Typhoid, I: Influenza.
DISCUSSION
This seroepidemiological investigation in health-care centers in Lagos, Nigeria, revealed a substantial burden of pediatric influenza infection and coinfection of influenza A with malaria parasitemia and typhoid fever. To the best of our knowledge, this is the first study to be reported on coinfection of the trio of influenza A, malaria parasitemia, and typhoid fever. Coinfected children reported the highest number of chest pain and high number of cold/chill symptom compared to children having influenza A monoinfection. Interestingly, coinfection was found in children of all age groups and all through the entire months of study. This study brings to the fore the burden of influenza infection as a cause of acute illness in these age groups and the need for influenza surveillance in health facilities, especially in children with influenza-like illness or pyrexia.
In the current study, a low seroprevalence of influenza A monoinfection was detected, similar to the prevalence of influenza infection in children previously reported.[30] However, when those with coinfection were included, the burden of influenza among these Nigerian population was quite high and similar to a previous study in Germany where seroprevalence to influenza A was tested using ELISA technique.[31] This is important considering that influenza is not routinely tested in Nigerian hospitals and most febrile illnesses are treated as malaria or typhoid fever. Other studies have also reported lower seroprevalence of 11.6% and 14.9%, and 15.8%, respectively, to influenza A virus in children.[32-34] The higher prevalence in this study may be related to increased exposure to predisposing factors for influenza among Nigerian children who often live in overcrowded conditions, suffer malnutrition and poverty.
This present study shows that coinfection with influenza A and malaria parasitemia (61.8%) was higher than that of influenza virus and typhoid fever (55.3%). The rate of coinfection with malaria is, however, lower than 92.2% coinfection rate reported among children in Uganda.[35] This could be attributed to the high prevalence of malaria in Nigeria regardless of influenza with more than two-third of malaria cases in sub-Saharan Africa occurring in children in Nigeria and accounting for 60% of outpatient visits and 30% childhood mortality.[19,20] However, our finding of influenza A and malaria coinfection was higher compared to the rare coinfection of malaria and influenza in Kenya.[13]
Interestingly, age group <0.5 year accounted for the highest burden of influenza coinfection with malaria parasitemia and typhoid fever compared to influenza alone. Furthermore, greater burden of influenza A with malaria and influenza A with typhoid fever coinfections, respectively, was detected in children aged 0.5–1 year compared to influenza A monoinfection. These findings are important because coinfection of influenza with malaria in these very young children often leads to more severe illness which may be due to complications of influenza such as bronchiolitis, laryngotracheobronchitis (croup), and tracheitis.[36] Therefore, screening, identification, and treatment of influenza under such circumstances is paramount and has the potential to improve outcome. The prevalence of influenza among young children in Africa (under-5 years) is higher than that in developed countries and similar to the prevalence of malaria and typhoid fever in this age group.[7,37-39] This may suggest shared exposure to risk factors to all three conditions which are underscored by the social determinants of health. Studies from other developing countries such as India and Bangladesh corroborate these findings.[38,39] Developed countries also have the highest frequency of influenza under the age of 5 years as shown from the American Center for Disease Control (CDC) and elsewhere.[40,41]
The male children in this study had higher IgM seroprevalence to influenza A virus similar to the finding reported in Mozambique during the 2015 season.[11] This was also supported by the 2012–2013 influenza season outcomes in Italy.[42] However, gender disparity to influenza observed in this current study was not statistically significant and alludes to the fact that children irrespective of their gender were infected with influenza A and can easily spread the virus due to their poor hygiene practices including lack of good respiratory hygiene during coughing and sneezing.
Influenza activity was confirmed throughout the months in which samples were collected with a higher occurrence recorded in the month of March. This is in line with the findings in Mozambique which showed that influenza occurred throughout the study interval but the activity was peak in February and August.[11] In contrast to this study, investigation in Burkina Faso showed that influenza was most prevalent in September–October but also occurred all year round.[43] Year-round transmission of influenza in Nigeria and other tropical countries is important with regard to vaccination programs in this regions. It implies that vaccination for influenza to these regions should be conducted all year round based on the available vaccine composition. It also calls for further studies to map the circulating viruses across these regions and their susceptibility to the available vaccines.
Previous studies have shown correlation between influenza, sickle cell disease, and asthma, but we did not find a high frequency of influenza among these children in the current study.[10,14] However, the high frequency of sickle cell disease in Nigeria calls for prioritization of influenza vaccination among children as well as prevention of malaria and typhoid fever to prevent severe complications.[44]
A recognized limitation in this study is the inability to further analyze the ELISA positive samples by molecular assay due to limited resources. Therefore, there is a need for a future study that can generate nucleotide sequences for global data sharing and possible consideration as vaccine candidates from sub-Saharan Africa.
CONCLUSION
Our investigation revealed specific IgM antibody seroprevalence to influenza A virus of 20.9% among children and 27.6% in children with influenza having coinfection of malaria and typhoid fever. Noteworthy was a likely year-round occurrence of the virus and more symptoms among children with malaria who were coinfected with influenza. This information is important for public health planning as it brings to the fore the high burden of influenza infection in a tropical country where the diagnosis of influenza is rarely made and routine influenza immunization is not currently practiced.
Acknowledgments
We acknowledge the Directorate of Clinical Services and Training/Hospital Research Ethics Committee of the Lagos State University Teaching Hospital for their support. We are grateful to the management and staff of the health centers where samples were collected, especially parents that volunteered and consented that their children should be recruited into the study. We acknowledge all the technologists that supported the research work. Many thanks to Dr. MI Kazeem, a senior faculty member in the department of biochemistry for revising the manuscript. The abstract for this study was submitted for presentation at the 29th Annual Meeting of the Society for Virology at the Heinrich Heine University, Dusseldorf, Germany, in March 20–23, 2019.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Molecular characterization of influenza A (H1N1) pdm09 in Cameroon during the 2014-2016 influenza seasons. PLoS One. 2019;141:e0210119.
- [CrossRef] [PubMed] [Google Scholar]
- Immunogenicity and safety of the new inactivated quadrivalent influenza vaccine vaxigrip tetra: Preliminary results in children =6 months and older adults. Vaccines (Basel). 2018;6:14.
- [CrossRef] [PubMed] [Google Scholar]
- Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2018;391:1285-300.
- [CrossRef] [Google Scholar]
- WHO Recommendation on the Composition of Influenza Virus Vaccines. 2018. Geneva: World Health Organization; Available from: http://www.who.int/influenza/vaccines/virus/recommendations/en/index.html [Last accessed on 2018 Dec 08]
- [Google Scholar]
- Pandemic influenza A (2009 H1N1) in children with acute lymphoblastic leukaemia. Br J Haematol. 2010;1496:874-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of influenza in children. Infect Dis Clin North Am. 2015;29:597-615.
- [CrossRef] [PubMed] [Google Scholar]
- Global role and burden of influenza in pediatric respiratory hospitalizations, 1982-2012. A systematic analysis. PLoS Med. 2016;133:e1001977.
- [Google Scholar]
- Influenza (Seasonal) Clinical Signs and Symptoms. 2020. Geneva: World Health Organization; Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) [Last accessed on 2020 May 03]
- [Google Scholar]
- Seasonal influenza virus infection among unvaccinated pregnant women in Lagos, Nigeria. Int J Infect Dis. 2018;73:368.
- [CrossRef] [Google Scholar]
- Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125:234-43.
- [CrossRef] [PubMed] [Google Scholar]
- Antigenic and genetic characterization of influenza viruses isolated in Mozambique during the 2015 season. PLoS One. 2018;13:e0201248.
- [CrossRef] [PubMed] [Google Scholar]
- Influenza virus infection is associated with increased risk of death amongst patients hospitalized with confirmed pulmonary tuberculosis in South Africa, 2010-2011. BMC Infect Dis. 2015;15:26.
- [CrossRef] [PubMed] [Google Scholar]
- Influenza and malaria coinfection among young children in Western Kenya, 2009-2011. J Infect Dis. 2012;206:1674-84.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of influenza vaccines in asthma: A systematic review and meta-analysis. Clin Infect Dis. 2017;65:1388-95.
- [CrossRef] [PubMed] [Google Scholar]
- Status of artemisinin resistance in malaria parasite plasmodium falciparum from molecular analyses of the Kelch13 gene in Southwestern Nigeria. Biomed Res Int. 2018;2018:2305062.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of malaria in children between the ages 2-15 visiting Gwarinpa general hospital life-camp, Abuja, Nigeria. J Health Sci. 2015;53:47-51.
- [Google Scholar]
- Is Nigeria winning the battle against malaria? Prevalence, risk factors and KAP assessment among Hausa communities in Kano state. Malar J. 2016;15:351.
- [CrossRef] [PubMed] [Google Scholar]
- WHO Global Malaria Report 2015. 2015. Geneva, Switzerland: World Health Organization; Available from: https://www.who.int/malaria/publications/world-malaria-report-2015/report/en [Last accessed on 2020 Sep 02]
- [Google Scholar]
- Malaria prevalence, anemia and baseline intervention coverage prior to mass net distributions in Abia and Plateau states, Nigeria. BMC Infect Dis. 2014;14:168.
- [CrossRef] [PubMed] [Google Scholar]
- Spatio-temporal trends of typhoid fever among youths attending Muhammad Abdullahi wase specialist hospital in Kano Metropolis, Nigeria. Bayero J Pure Appl Sci. 2017;10:115-21.
- [CrossRef] [Google Scholar]
- Typhoid fever: Tracking the trend in Nigeria. Am J Trop Med Hyg. 2018;99(Suppl 3):41-7.
- [CrossRef] [PubMed] [Google Scholar]
- Implementation of interventions for the control of typhoid fever in low-and middle-income countries. Am J Trop Med Hyg. 2018;99(Suppl 3):79-88.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of sensitivity and specificity of ELISA against Widal test for typhoid diagnosis in endemic population of Kathmandu. BMC Infect Dis. 2015;15:523.
- [CrossRef] [PubMed] [Google Scholar]
- Influenza viruses in Nigeria, 2009-2010. Results from the first 17 months of a national influenza sentinel surveillance system. J Infect Dis. 2012;206(Suppl 1):S121-8.
- [CrossRef] [PubMed] [Google Scholar]
- High seroprevalence of influenza virus among pig-handlers/swine workers in Lagos, Nigeria. LASU J Eng Sci Tech. 2019;1:48-53.
- [Google Scholar]
- Viral etiology of acute respiratory infections in hospitalized children in Novosibirsk city, Russia 2013-2017. PLoS One. 2018;13:e0200117.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of antibodies against influenza A and B viruses in children in Germany, 2008 to 2010. Euro Surveill. 2014;195:20687.
- [CrossRef] [PubMed] [Google Scholar]
- Influenza virus infection among pediatric patients reporting diarrhea and influenza-like illness. BMC Infect Dis. 2010;10:3.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship of influenza virus infection to associated infections in children who present with influenza-like symptoms. BMC Infect Dis. 2016;16:304.
- [CrossRef] [PubMed] [Google Scholar]
- Seroprevalence of influenza A and B viruses among unvaccinated children in the United Arab Emirates: A cross-sectional study. BMC Res Notes. 2017;10:379.
- [CrossRef] [PubMed] [Google Scholar]
- Co-infection of malaria and influenza viruses in Uganda: A pilot study. Int J Infect Dis. 2014;21:1-460.
- [CrossRef] [Google Scholar]
- Clinical Signs and Symptoms of Influenza. 2019. Available from: https://www.cdc.gov/flu/professionals/acip/clinical.htm [Last accessed on 2020 Aug 29]
- [Google Scholar]
- Risk factors for influenza-associated severe acute respiratory illness hospitalization in South Africa, 2012-2015. Open Forum Infect Dis. 2017;4:ofw262.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of influenza-associated hospitalization in rural communities in western and Northern India, 2010-2012. A multi-site population-based study. J Infect. 2015;702:160-70.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of respiratory virus-associated pneumonia in urban poor young children of Dhaka, Bangladesh, 2009-2011. PLoS One. 2012;7:e32056.
- [CrossRef] [PubMed] [Google Scholar]
- Estimates of deaths associated with seasonal influenza 1976-2007. MMWR Morb Mortal Wkly Rep. 2013;59:1057-62.
- [Google Scholar]
- Influenza pneumonia: A comparison between seasonal influenza virus and the H1N1 pandemic. Eur Respir J. 2011;38:106-11.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features of children hospitalized with influenza A and B infections during the 2012-2013 influenza season in Italy. BMC Infect Dis. 2015;16:66.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and molecular characterization of influenza viruses in Burkina Faso, Sub-Saharan Africa. Influenza Other Respir Viruses. 2018;12:490-6.
- [CrossRef] [PubMed] [Google Scholar]
- Management of sickle cell disease: A review for physician education in Nigeria (Sub-Saharan Africa) Anemia. 2015;2015:791498.
- [CrossRef] [PubMed] [Google Scholar]






